Abstract
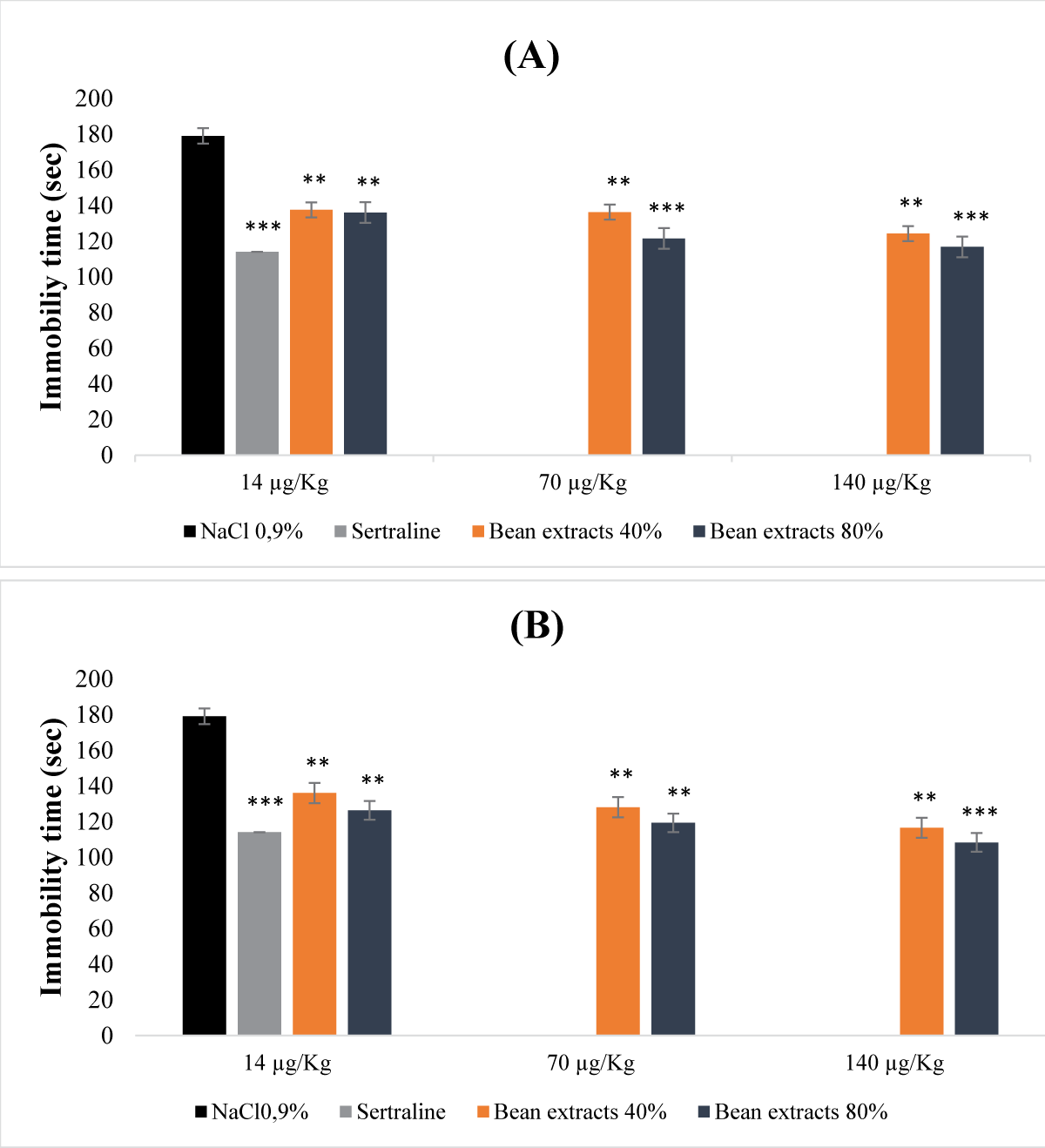
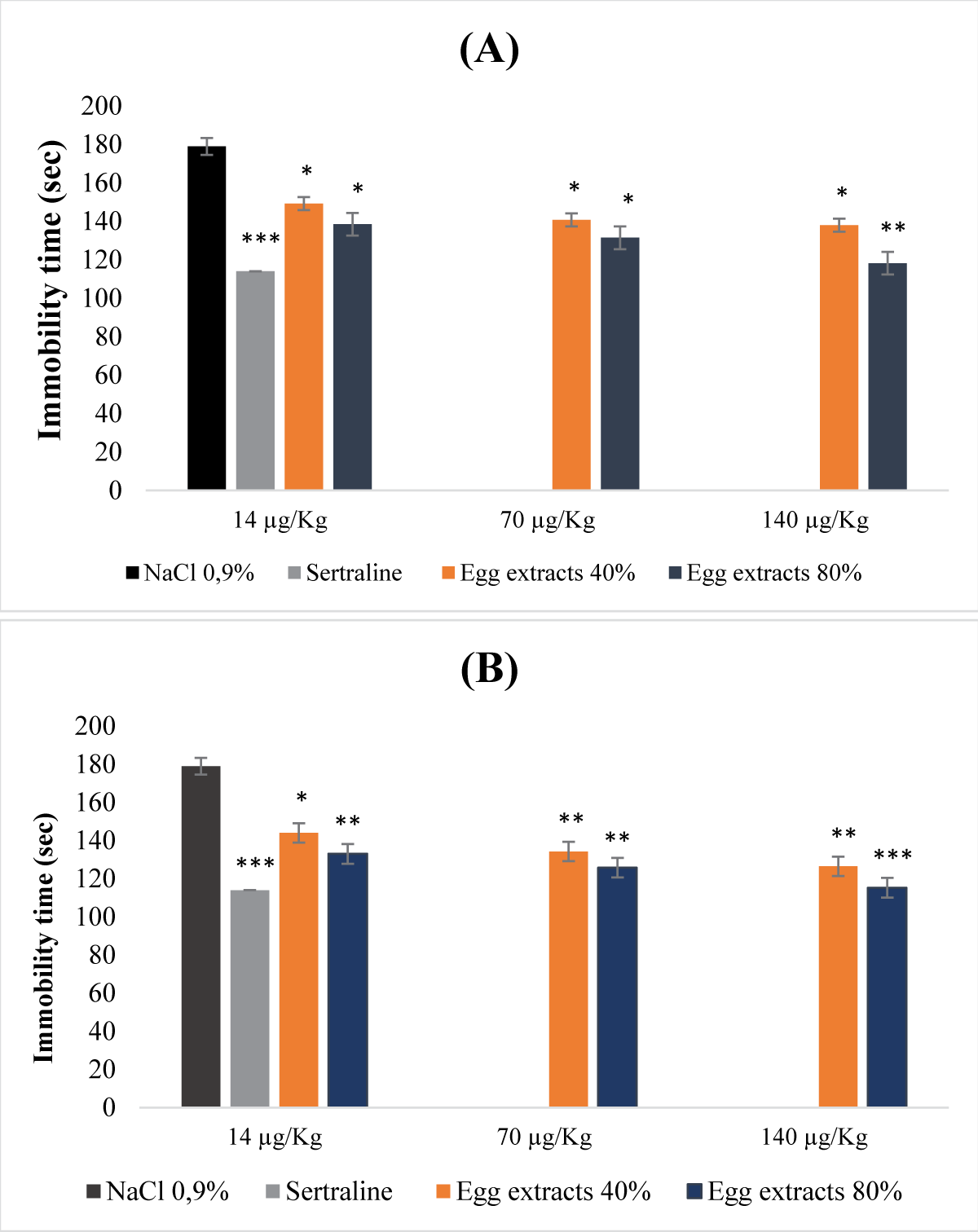
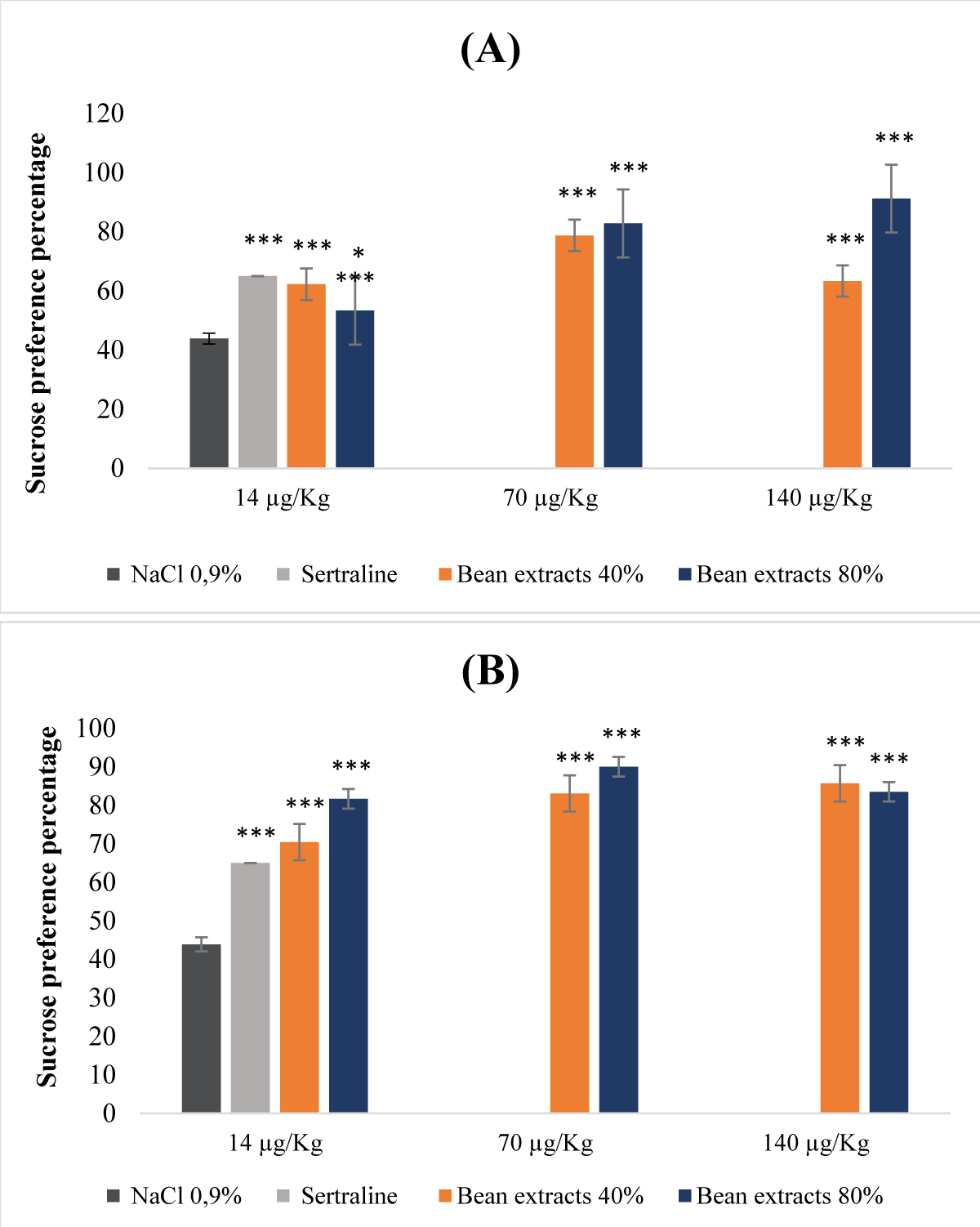
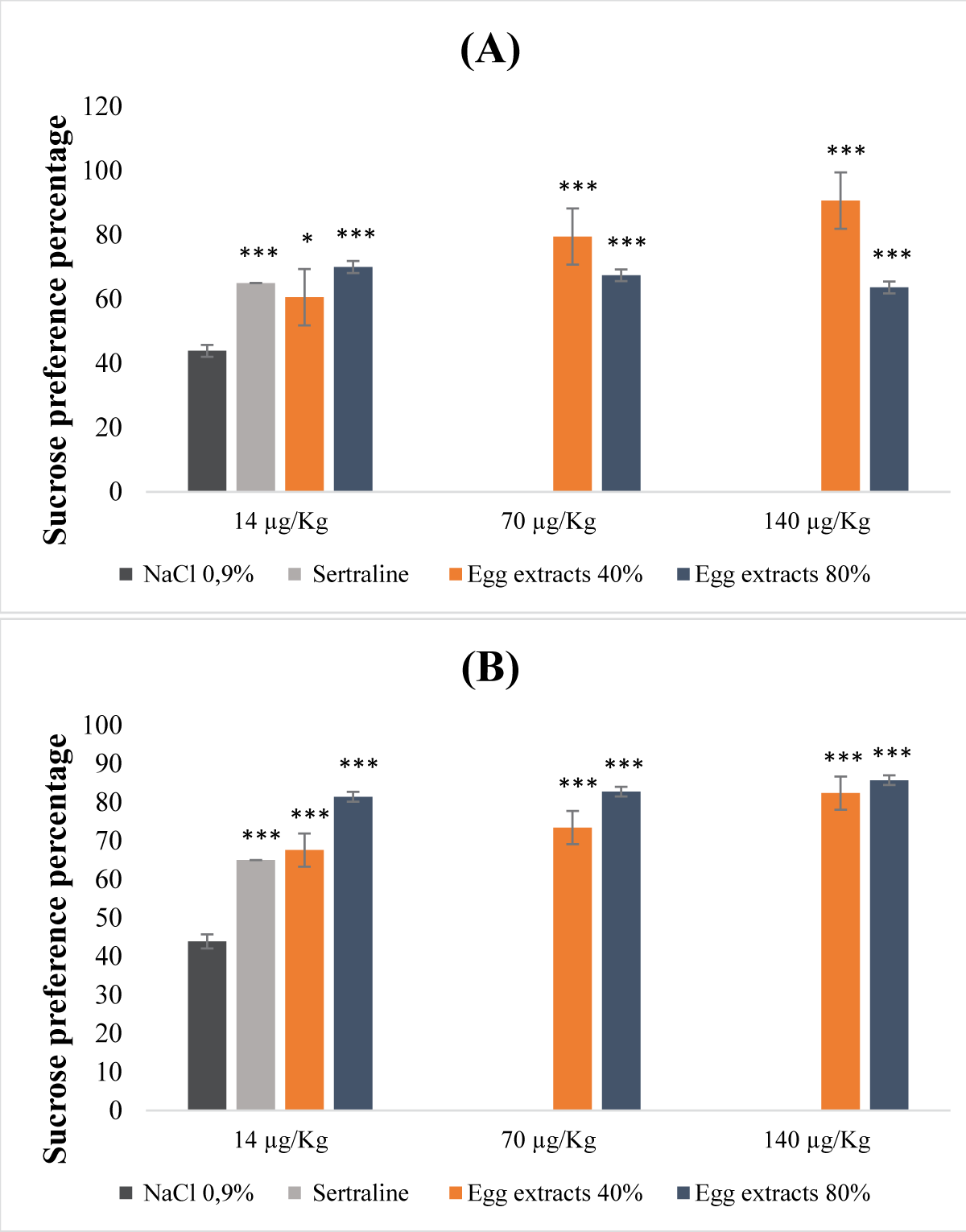
This research reveals the previously unexplored pharmacognostic potential of antidepressants found in nutrients derived from both legume and animal sources. Through preclinical investigations involving mouse models, the study focused into antidepressant and antioxidant activities of non-denatured and denatured protein extracts from beans and eggs. Non-denatured protein extracts from beans and eggs, at saturation levels of 40% and 80%, were examined as macronutrients, while denatured protein extracts at equivalent saturation levels were considered micronutrients. The study employed the DPPH and hydrogen peroxide tests to assess antioxidant activity, and the forced swimming test and sucrose preference test to evaluate acute and chronic mild antidepressant effects, respectively. The acute toxicity study revealed that macronutrients from eggs at 40% and 80% saturation displayed non-toxic effects (LD50 >5 g/kg), while those from beans, specifically at saturation of 80%, exhibited a relatively low level of toxicity (LD50 = 2.5 g/kg). Evaluation of antioxidant activity using the DPPH test yielded inconclusive results due to the influence of ethanol precipitation. In contrast, the H2O2 test demonstrated significant antioxidant potential in both macronutrients and micronutrients extracted from beans and eggs at all saturation levels. In investigating antidepressant properties, both macronutrients and micronutrients of bean and egg protein extracts at 40% and 80% saturation exhibited notable antidepressant effects, particularly the micronutrients at saturation of 80%. This antidepressant effect was characterized by a reduction in immobility time and an increase in sucrose preference.
In conclusion, this study uncovers the multifaceted potential of protein extracts sourced from natural products, plant and animal origins, as agents for treating depression. It opens up new avenues for research, with implications ranging from neuroprotection to the management of depression, inspiring optimism for innovative approaches to mental health treatment.