
Peritoneal Carcinomatosis from Ovarian Cancer: A Case Report
Oncology SurgeryPharmacologyReceived 14 Dec 2023 Accepted 03 May 2024 Published online 06 May 2024
ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
The Expressivity Dimension of Speech is the basis of the Expression Dimension. Evidence from Behavioural and Neuroimaging Studies
Previous Full Text
Wishful Thinking about Consciousness


Received 14 Dec 2023 Accepted 03 May 2024 Published online 06 May 2024
Epithelial ovarian cancer is one of the most common causes of cancer-related death in women. More than half of patients are diagnosed at an advanced stage, usually due to the locoregional spread of peritoneal carcinomatosis. We present the case of a 49-year-old woman who suffered from constitutional syndrome and abdominal distention. The imaging tests revealed two large, vascularized, and heterogeneous masses that depended on both ovaries. Bilateral adnexectomy was performed and the result of the anatomopathological study was endometrioid carcinoma moderately differentiated. The patient was treated according to the latest recommendations of the NCCN guidelines. A combination of systemic chemotherapy and cytoreductive surgery has been the standard treatment since the mid-1990s. However, a combination of hyperthermic intravenous and intraperitoneal chemotherapy may reduce plasma toxicity and increase therapeutic effectiveness.
Epithelial ovarian cancer is the leading cause of death from gynecologic cancer in Western countries, with less than half of patients living >5 years following diagnosis []. The absence of an effective screening program as well as specific symptoms, makes the diagnosis difficult and often made in advanced stages of the disease, in the presence of peritoneal dissemination. The complete cytoreduction of the disease and tumor sensitivity to systemic chemotherapy based on platinum, are the two main prognostic factors []. In the absence of other systemic metastases, multimodal approaches combining aggressive Cytoreductive Surgery (CRS), intraperitoneal Hyperthermic Chemotherapy (HIPEC), and systemic chemotherapy have been proposed and are considered promising methods to improve loco-regional control of the disease and ultimately to increase survival []. We report a clinical case of peritoneal carcinomatosis from ovarian cancer treated in this way.
A 49-year-old woman presented with diffuse abdominal pain, abdominal distension, and nausea, but no vomiting. She also associated loss of appetite and weight of 8 kg. She had no personal or family history of interest. The examination revealed a soft, depressible, distended, and non-painful abdomen.
The Computed Tomography (CT) scan reported two large, vascularized, and heterogeneous masses that depended on both ovaries, the one on the left side measured up to 16 x 10 cm and the other, 20 x 10 cm. She had ascitic fluid suggestive of peritoneal carcinomatosis. No mediastinal, pelvic, retroperitoneal, or mesenteric lymphadenopathy was seen. No figures are suggestive of metastasis were observed. Bilateral pleural effusion was observed (Figures 1-3). The levels of tumor markers were: Ca 125: 581 U/mL and HE-4: > 1500 pmol/L. The study was completed with Magnetic Resonance Imaging (MRI): large pelvic mass 21 x 26 x 10 cm with extension to the lower abdomen, probably of bilateral ovarian origin. The tumor contacts and displaces intestinal loops superiorly, as well as large retroperitoneal vessels. Exploratory laparotomy was performed; finding abundant ascites (1500cc) that were aspirated and sent for histological study, the ovarian masses described in the imaging tests, implants on both diaphragmatic domes in miliary seeding, as well as that on both abdominal flanks, ligament falciform, intestinal loops, omentum, uterus, bladder peritoneum, and Douglas cul-de-sac. PCI: 21. Bilateral adnexectomy was performed. The result of the anatomopathological study was: endometrioid carcinoma moderately differentiated, G2, bilateral (20 x 12 cm right side and 23 x 14 cm left side) with BCRA 1 and 2 wild type.
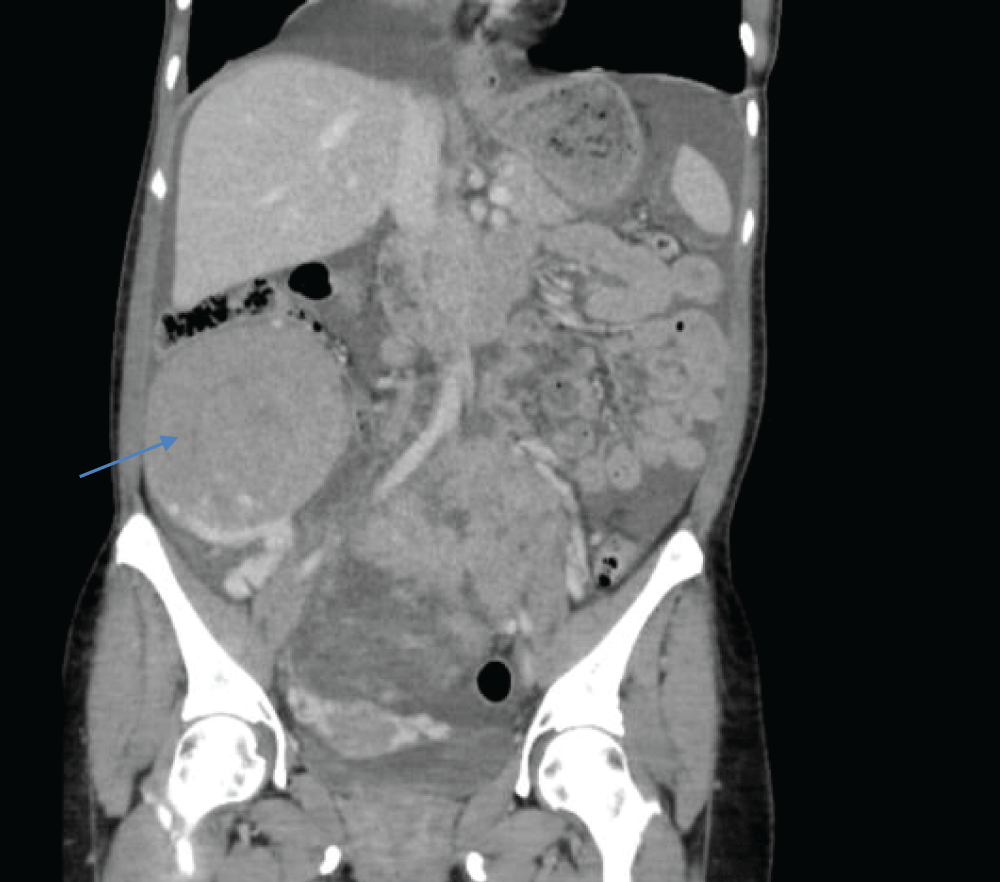 Figure 1: The tumor (→) contacts and displaces intestinal loops superiorly, as well as large retroperitoneal vessels.
Figure 1: The tumor (→) contacts and displaces intestinal loops superiorly, as well as large retroperitoneal vessels.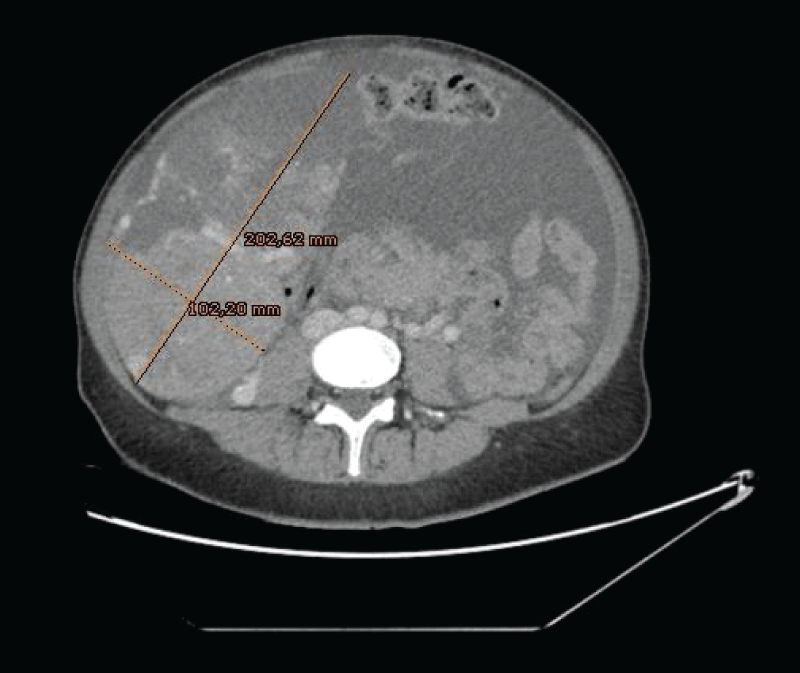 Figure 2: Large, vascularized, and heterogeneous masses that depended on both ovaries, the one on the right side measured 20 x 10 cm.
Figure 2: Large, vascularized, and heterogeneous masses that depended on both ovaries, the one on the right side measured 20 x 10 cm.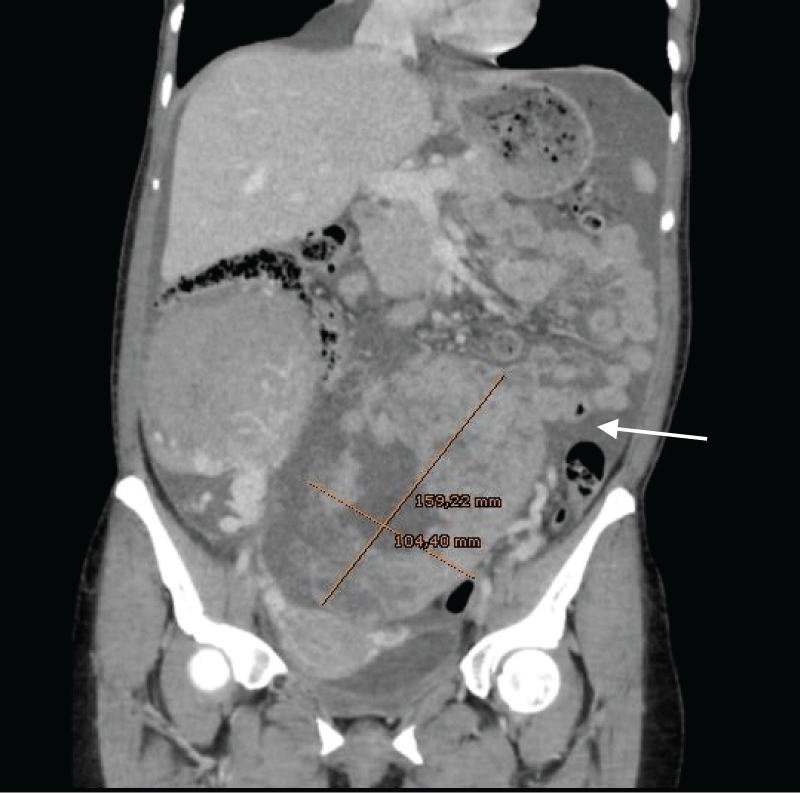 Figure 3: Ascitic fluid suggestive of peritoneal carcinomatosis (→). Large, vascularized, and heterogeneous masses that depended on both ovaries, the one on the left side measured up to 16 x 10 cm.
Figure 3: Ascitic fluid suggestive of peritoneal carcinomatosis (→). Large, vascularized, and heterogeneous masses that depended on both ovaries, the one on the left side measured up to 16 x 10 cm.Therefore, it was a 49-year-old patient diagnosed with endometrioid carcinoma ovarian stage IIIC and probable stage IVa due to pleural effusion (no cytology was taken, so she cannot be classified as M1a). Neoadjuvant chemotherapy started with Carboplatin + Paclitaxel and after 3 cycles of treatment it was reported a favorable radiological evolution (Figure 4,5), so interval cytoreductive surgery+ HIPEC (with Cisplatin and bicavera, 60 minutes, 42 ºC) was performed.
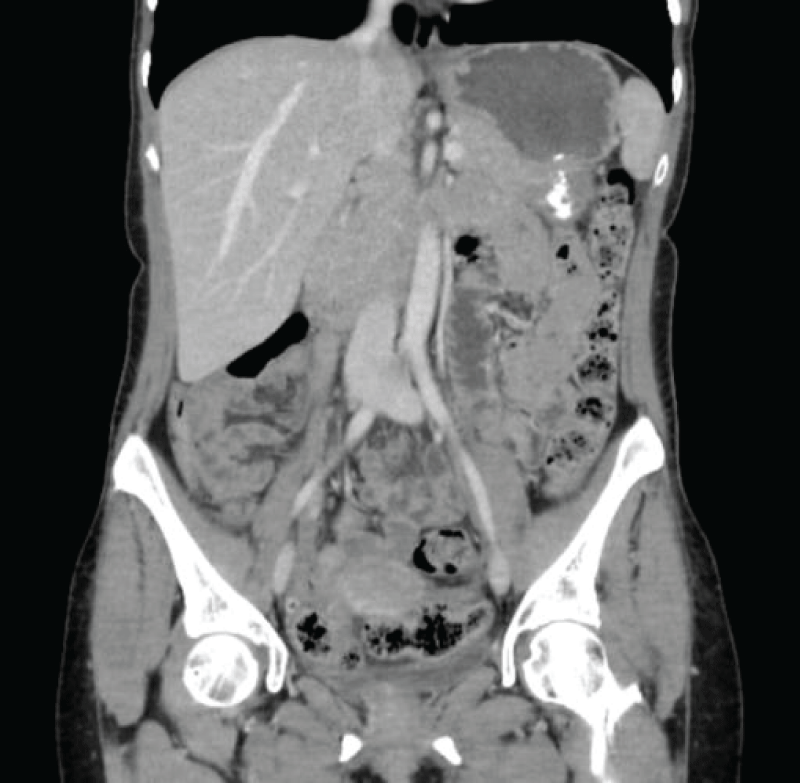 Figure 4: Postsurgical changes in the pelvic area concerning bilateral oophorectomy. Complete disappearance of previously existing ascites.
Figure 4: Postsurgical changes in the pelvic area concerning bilateral oophorectomy. Complete disappearance of previously existing ascites.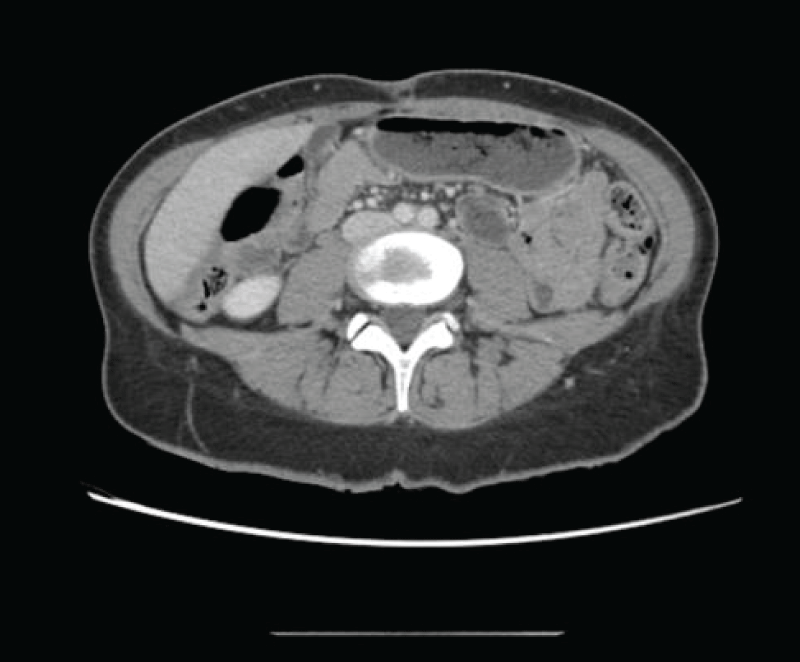 Figure 5: Subtly granular appearance of the peritoneal fat, which does not allow to conclusively rule out metastatic carcinomatous dissemination.
Figure 5: Subtly granular appearance of the peritoneal fat, which does not allow to conclusively rule out metastatic carcinomatous dissemination.The postoperative evolution was favorable, with good pain control, good oral tolerance, and positive intestinal transit at discharge. The CT scan two months after the intervention showed no signs of recurrence or distant spread.
The microscopic component of the disease at the end of the cytoreduction is responsible for these recurrences and the use of intraoperative Hyperthermic Intraperitoneal Chemotherapy (HIPEC) at the same time of surgery has been proposed as a reasonable therapeutic option for treatment []. Peritonectomy procedures used in cytoreductive surgery for peritoneal malignancy are Glisson’s capsulectomy, omentectomy, left and right diaphragmatic peritonectomy, lesser omentectomy, stripping of the omental bursa, and pelvic peritonectomy []. It can be accompanied by hysterectomy, bilateral adnexectomy, and intestinal resection. In our case, bilateral adnexectomy had previously been performed.
HIPEC privileges consist of increasing loco-regional drug concentration limiting their systemic diffusion and consequentially their toxicity and adverse events. The role of the peritoneal plasma barrier in promoting a loco-regional high-dose effect is very important. Indeed, the peritoneum can limit systemic drug diffusion in the peritoneal space. Moreover, hyperthermia enhances the efficacy and penetration of many of the drugs employed [].
The randomized clinical trial initiated in 2010 by Lim, et al. was conducted to assess the clinical benefit of HIPEC after primary or interval maximal cytoreductive surgery in women with stage III or IV primary advanced ovarian cancer. The conclusions were that the addition of HIPEC after interval cytoreductive surgery following neoadjuvant chemotherapy reduced the recurrence and mortality rates in women with primary stage III or IV epithelial ovarian cancer. However, the survival benefit of HIPEC immediately after primary cytoreductive surgery was not identified [].
The great advance has been the publication of the Dutch clinical trial (NCT00426257) in 2019. 245 patients were randomized in the setting of interval cytoreductive surgery after neoadjuvant chemotherapy (NACT). Among patients with stage III epithelial ovarian cancer, the addition of HIPEC to interval cytoreductive surgery resulted in longer recurrence-free survival and overall survival than surgery alone and did not result in higher rates of side effects []. This study has marked a turning point, so HIPEC now appears in oncological guidelines as a clear treatment option. Along these lines, the ESMO-ESGO systematic review and meta-analysis published in 2022 concludes that HIPEC after neoadjuvant chemotherapy significantly increases 5-year overall survival and disease-free survival in advanced primary ovarian cancer [].
This complex approach to peritoneal metastases has offered very high survival rates compared to single chemotherapy treatment, which is why its implementation and development are becoming increasingly widespread in hospitals around the world. With this, our advanced oncological surgery unit treats peritoneal carcinomatosis of ovarian and colorectal origin, as well as peritoneal pseudomyxoma. In the case of ovarian cancer, women who suffer from this type of tumor present an extension in the form of peritoneal metastases in up to 75% of cases. In these patients, whose alternative recently was palliative treatment, survival rates of up to 70% have been achieved after 5 years and a median overall survival of 70 months. This is a very positive result if we take into account the Dutch clinical trial study (NCT00426257), whose results were 50% of patients died at a median follow-up of 4.7 years and a median overall survival of 45.7 months in the surgery-plus- HIPEC group [].
Because of positive results, the NCCN Guidelines now include an option to consider HIPEC at the time in patients with stage III disease treated with NACT. Similar to the trial, the NCCN Guidelines recommend HIPEC as an option for patients who have a response or stable disease after NACT (3 cycles preferred, but 4–6 allowed) and that all patients treated with NACT and cytoreductive surgery (± HIPEC) receive postoperative chemotherapy [].
Epithelial ovarian cancer is one of the most common causes of cancer-related death in women. More than half of patients are diagnosed at an advanced stage, usually due to the locoregional spread of peritoneal carcinomatosis. A combination of systemic chemotherapy and cytoreductive surgery has been the standard treatment since the mid-1990s. However, conventional chemotherapy is poorly delivered to the peritoneum due to the plasma-peritoneal barrier. Intraperitoneal chemotherapy can improve survival by eliminating residual microscopic disease. A combination of hyperthermic intravenous and intraperitoneal chemotherapy may reduce plasma toxicity and increase therapeutic effectiveness.
Written informed consent was obtained from the patient as a part of institutional standard practice. IRB ethical approval was received before drafting the manuscript.
Armstrong DK, Alvarez RD, Backes FJ, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, Chen LM, Chitiyo VC, Cristea M, DeRosa M, Eisenhauer EL, Gershenson DM, Gray HJ, Grisham R, Hakam A, Jain A, Karam A, Konecny GE, Leath CA III, Leiserowitz G, Liu J, Martin L, Matei D, McHale M, McLean K, Miller DS, Percac-Lima S, Remmenga SW, Schorge J, Stewart D, Thaker PH, Vargas R, Hendrickson AW, Werner TL, Zsiros E, Dwyer MA, Hang L. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022. J Natl Compr Canc Netw. 2022 Sep;20(9):972-980. doi: 10.6004/jnccn.2022.0047. PMID: 36075393.
Cascales-Campos P, Gil J, Feliciangeli E, Parrilla P. HIPEC in ovarian cancer: treatment of a new era or is it the end of the pipeline? Gynecol Oncol. 2015 Nov;139(2):363-8. doi: 10.1016/j.ygyno.2015.06.012. Epub 2015 Jun 16. PMID: 26091936.
Coccolini F, Gheza F, Lotti M, Virzì S, Iusco D, Ghermandi C, Melotti R, Baiocchi G, Giulini SM, Ansaloni L, Catena F. Peritoneal carcinomatosis. World J Gastroenterol. 2013 Nov 7;19(41):6979-94. doi: 10.3748/wjg.v19.i41.6979. PMID: 24222942; PMCID: PMC3819534.
Mercier F, Mohamed F, Cazauran JB, Kepenekian V, Vaudoyer D, Cotte E, Glehen O, Passot G. An update of peritonectomy procedures used in cytoreductive surgery for peritoneal malignancy. Int J Hyperthermia. 2019;36(1):744-752. doi: 10.1080/02656736.2019.1635717. PMID: 31401893.
Lim MC, Chang SJ, Park B, Yoo HJ, Yoo CW, Nam BH, Park SY; HIPEC for Ovarian Cancer Collaborators. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022 May 1;157(5):374-383. doi: 10.1001/jamasurg.2022.0143. PMID: 35262624; PMCID: PMC8908225.
van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT, van der Velden J, Arts HJ, Massuger LFAG, Aalbers AGJ, Verwaal VJ, Kieffer JM, Van de Vijver KK, van Tinteren H, Aaronson NK, Sonke GS. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan 18;378(3):230-240. doi: 10.1056/NEJMoa1708618. PMID: 29342393.
Filis P, Mauri D, Markozannes G, Tolia M, Filis N, Tsilidis K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: a systematic review and meta-analysis of randomized trials. ESMO Open. 2022 Oct 1 [cited 2024 May 2];7(5). https://www.esmoopen.com/article/S2059-7029(22)00216-2/fulltext
Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, Berek JS, Chen LM, Cristea M, DeRosa M, ElNaggar AC, Gershenson DM, Gray HJ, Hakam A, Jain A, Johnston C, Leath CA III, Liu J, Mahdi H, Matei D, McHale M, McLean K, O'Malley DM, Penson RT, Percac-Lima S, Ratner E, Remmenga SW, Sabbatini P, Werner TL, Zsiros E, Burns JL, Engh AM. NCCN Guidelines Insights: Ovarian Cancer, Version 1.2019. J Natl Compr Canc Netw. 2019 Aug 1;17(8):896-909. doi: 10.6004/jnccn.2019.0039. PMID: 31390583.
Godos AGD, Diaz EN, Fuentes PI, Saborido BA, Sánchez DA. Peritoneal Carcinomatosis from Ovarian Cancer: A Case Report. IgMin Res. May 06, 2024; 2(5): 309-312. IgMin ID: igmin181; DOI:10.61927/igmin181; Available at: igmin.link/p181
Anyone you share the following link with will be able to read this content:
Address Correspondence:
Andrea González De Godos, Department of General Surgery and Digestive System, Río Hortega University Hospital, Valladolid, Spain, Email: [email protected]
How to cite this article:
Godos AGD, Diaz EN, Fuentes PI, Saborido BA, Sánchez DA. Peritoneal Carcinomatosis from Ovarian Cancer: A Case Report. IgMin Res. May 06, 2024; 2(5): 309-312. IgMin ID: igmin181; DOI:10.61927/igmin181; Available at: igmin.link/p181
Copyright: © 2024 De Godos AG, et al This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 Figure 1: The tumor (→) contacts and displaces intestinal ...
Figure 1: The tumor (→) contacts and displaces intestinal ...
 Figure 2: Large, vascularized, and heterogeneous masses that...
Figure 2: Large, vascularized, and heterogeneous masses that...
 Figure 3: Ascitic fluid suggestive of peritoneal carcinomato...
Figure 3: Ascitic fluid suggestive of peritoneal carcinomato...
 Figure 4: Postsurgical changes in the pelvic area concerning...
Figure 4: Postsurgical changes in the pelvic area concerning...
 Figure 5: Subtly granular appearance of the peritoneal fat, ...
Figure 5: Subtly granular appearance of the peritoneal fat, ...
Armstrong DK, Alvarez RD, Backes FJ, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, Chen LM, Chitiyo VC, Cristea M, DeRosa M, Eisenhauer EL, Gershenson DM, Gray HJ, Grisham R, Hakam A, Jain A, Karam A, Konecny GE, Leath CA III, Leiserowitz G, Liu J, Martin L, Matei D, McHale M, McLean K, Miller DS, Percac-Lima S, Remmenga SW, Schorge J, Stewart D, Thaker PH, Vargas R, Hendrickson AW, Werner TL, Zsiros E, Dwyer MA, Hang L. NCCN Guidelines® Insights: Ovarian Cancer, Version 3.2022. J Natl Compr Canc Netw. 2022 Sep;20(9):972-980. doi: 10.6004/jnccn.2022.0047. PMID: 36075393.
Cascales-Campos P, Gil J, Feliciangeli E, Parrilla P. HIPEC in ovarian cancer: treatment of a new era or is it the end of the pipeline? Gynecol Oncol. 2015 Nov;139(2):363-8. doi: 10.1016/j.ygyno.2015.06.012. Epub 2015 Jun 16. PMID: 26091936.
Coccolini F, Gheza F, Lotti M, Virzì S, Iusco D, Ghermandi C, Melotti R, Baiocchi G, Giulini SM, Ansaloni L, Catena F. Peritoneal carcinomatosis. World J Gastroenterol. 2013 Nov 7;19(41):6979-94. doi: 10.3748/wjg.v19.i41.6979. PMID: 24222942; PMCID: PMC3819534.
Mercier F, Mohamed F, Cazauran JB, Kepenekian V, Vaudoyer D, Cotte E, Glehen O, Passot G. An update of peritonectomy procedures used in cytoreductive surgery for peritoneal malignancy. Int J Hyperthermia. 2019;36(1):744-752. doi: 10.1080/02656736.2019.1635717. PMID: 31401893.
Lim MC, Chang SJ, Park B, Yoo HJ, Yoo CW, Nam BH, Park SY; HIPEC for Ovarian Cancer Collaborators. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022 May 1;157(5):374-383. doi: 10.1001/jamasurg.2022.0143. PMID: 35262624; PMCID: PMC8908225.
van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT, van der Velden J, Arts HJ, Massuger LFAG, Aalbers AGJ, Verwaal VJ, Kieffer JM, Van de Vijver KK, van Tinteren H, Aaronson NK, Sonke GS. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018 Jan 18;378(3):230-240. doi: 10.1056/NEJMoa1708618. PMID: 29342393.
Filis P, Mauri D, Markozannes G, Tolia M, Filis N, Tsilidis K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: a systematic review and meta-analysis of randomized trials. ESMO Open. 2022 Oct 1 [cited 2024 May 2];7(5). https://www.esmoopen.com/article/S2059-7029(22)00216-2/fulltext
Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, Berek JS, Chen LM, Cristea M, DeRosa M, ElNaggar AC, Gershenson DM, Gray HJ, Hakam A, Jain A, Johnston C, Leath CA III, Liu J, Mahdi H, Matei D, McHale M, McLean K, O'Malley DM, Penson RT, Percac-Lima S, Ratner E, Remmenga SW, Sabbatini P, Werner TL, Zsiros E, Burns JL, Engh AM. NCCN Guidelines Insights: Ovarian Cancer, Version 1.2019. J Natl Compr Canc Netw. 2019 Aug 1;17(8):896-909. doi: 10.6004/jnccn.2019.0039. PMID: 31390583.