
A Review inside Innovation: AI and Additive Manufacturing for Advanced Bone Scaffold Design
BioengineeringReceived 27 Jul 2025 Accepted 23 Sep 2025 Published online 24 Sep 2025
ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
Clustering of Three-dimensional (3-D) Objects by Means of Phase- only Digital Holographic Information using Machine Learning
Previous Full Text
In What Way Does Climate Change Matter!?


Received 27 Jul 2025 Accepted 23 Sep 2025 Published online 24 Sep 2025
Integrating artificial intelligence (AI) and additive manufacturing (AM) has significantly transformed the design and fabrication of bone scaffolds, offering remarkable potential for advancing regenerative medicine and sustainable healthcare solutions. This paper examines how AI-driven generative design and predictive modeling enable the creation of customized bone scaffolds with superior mechanical properties, optimized porosity, and enhanced biocompatibility explicitly tailored to individual patient needs. Additive manufacturing complements these advancements by providing precise, waste-minimizing fabrication methods, while AI further supports the process through defect detection, optimization, and strategic material selection.
Recent innovations in innovative biomaterials, IoT-enabled implants, and closed-loop manufacturing systems have further amplified the effectiveness and sustainability of these scaffolds. The review emphasizes how the convergence of AI and AM technologies can substantially reduce production costs, enhance accessibility in resource-limited areas, and address pressing global healthcare challenges, as illustrated in Figure 1, which maps the primary challenges and technological advancements in scaffold design. Despite existing hurdles, including the high initial costs and environmental concerns associated with energy-intensive AI model training, these barriers can be mitigated through collaborative interdisciplinary research and innovation.
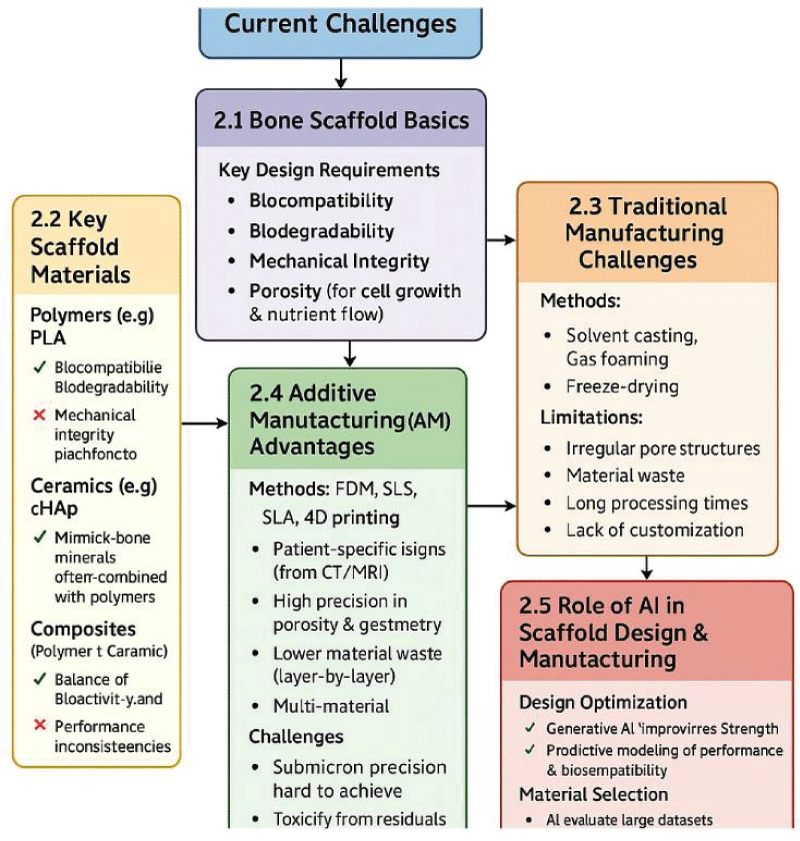 Figure 1: Flowchart illustrating the major challenges in traditional scaffold design and the technological advances provided by AI and additive manufacturing.
Figure 1: Flowchart illustrating the major challenges in traditional scaffold design and the technological advances provided by AI and additive manufacturing.This review highlights the profound potential of integrating AI and additive manufacturing in bone tissue engineering, underscoring their ability to provide scalable, personalized healthcare solutions aligned with global sustainability goals. Continued research, enhanced cross-disciplinary partnerships, and supportive policies are essential to realize and broadly disseminate these technological advancements.
The integration of artificial intelligence (AI) and additive manufacturing (AM) has significantly transformed bone scaffold design and production, demonstrating substantial advancements in regenerative medicine and sustainable healthcare [,]. Bone scaffolds play a crucial role in tissue engineering, providing temporary frameworks that support the regeneration of bone tissues affected by injury or disease. Traditionally, scaffold production has relied on manufacturing methods such as solvent casting, freeze-drying, and gas foaming, which frequently encounter limitations including inconsistent pore structures, inadequate mechanical strength, and substantial material waste [-].
Recent advances combining AI-driven generative design techniques and predictive modeling with additive manufacturing address these constraints by enabling precise fabrication of scaffolds with patient-specific architectures, optimized porosity, improved mechanical integrity, and enhanced biocompatibility [,]. AI techniques, including machine learning and deep learning algorithms, facilitate precise control over scaffold parameters, thus significantly enhancing scaffold performance and clinical outcomes while simultaneously minimizing material waste and environmental impact [,].
Moreover, the advent of smart biomaterials, IoT-enabled implants, and closed-loop manufacturing systems has significantly expanded bone scaffolds' functional capabilities and sustainability. These innovations enable scaffolds not only to effectively support bone regeneration but also dynamically monitor biological conditions and respond adaptively to environmental stimuli, optimizing the healing process [,].
This review paper critically examines how integrating AI and AM technologies can enhance patient outcomes, reduce healthcare costs, and improve accessibility, especially within resource-limited regions, thereby addressing key global healthcare challenges [,]. Despite significant promise, barriers such as high implementation costs and the environmental impact of energy-intensive AI training models must be carefully managed. Overcoming these challenges necessitates interdisciplinary collaboration, open-source initiatives, supportive policy frameworks, and continued technological innovation [,]. By systematically evaluating these technologies and their integration, this paper underscores the potential for AI and AM to provide scalable, personalized, and sustainable healthcare solutions on a global scale.
Bone scaffolds are critical components in regenerative medicine, acting as temporary structures that support cell adhesion, proliferation, and differentiation for effective bone tissue regeneration. Successful bone scaffold design must meet several essential criteria, including biocompatibility, biodegradability, mechanical integrity, and appropriate porosity []. These characteristics ensure scaffolds facilitate cellular activities, integrate seamlessly with surrounding tissues, and degrade safely upon completion of bone regeneration.
Common materials employed for bone scaffold fabrication include polymers, ceramics, and composites. Polymers, notably polylactic acid (PLA), are extensively used due to their biocompatibility, ease of fabrication, and inherent biodegradability. Despite these advantages, PLA's relatively low mechanical strength restricts its application to non-load-bearing scenarios, requiring reinforcement or composite formation for enhanced mechanical performance [-].
Ceramics, particularly calcium hydroxyapatite (cHAp), closely mimic the mineral composition of natural bone, providing excellent osteoconductivity essential for scaffold performance. However, their inherent brittleness and the challenges of fabricating complex geometric structures limit their standalone application. Consequently, ceramic-polymer composites have been developed, strategically combining the beneficial mechanical properties of polymers and the bioactivity of ceramics to create highly functional scaffold systems [,]. Nevertheless, inconsistencies in composite performance due to variations in ceramic-polymer interactions remain a concern, requiring precise manufacturing controls [,].
Polyetheretherketone (PEEK) is an advanced alternative recognized for its excellent mechanical properties, making it suitable for load-bearing implants, including spinal and orthopedic applications. However, despite its mechanical robustness, PEEK exhibits limited bioactivity, necessitating extensive surface modification or the incorporation of bioactive coatings to promote cellular attachment and osseointegration [-]. Recent studies have highlighted both the promising clinical outcomes of bioactive PEEK modifications and the challenges in maintaining long-term coating integrity under physiological conditions [].
Traditional scaffold manufacturing methods, such as solvent casting, gas foaming, and freeze-drying, are widely employed but inherently limited. These processes often fail to produce uniform, well-controlled pore structures essential for efficient nutrient and oxygen transport and effective cellular infiltration [-]. The inconsistent porosity and non-interconnected pore networks resulting from these conventional approaches negatively impact scaffold performance and subsequent bone regeneration outcomes.
Additionally, these traditional methods typically involve significant material wastage, prolonged processing times, and limited potential for personalized scaffold fabrication. Customization, crucial for addressing patient-specific anatomical needs, is challenging due to the inherent limitations of these production methods [,]. Consequently, a shift towards advanced technologies, such as additive manufacturing (AM), is crucial for overcoming these constraints. AM facilitates precise and efficient scaffold customization, thereby addressing critical gaps in the performance, sustainability, and patient specificity that traditional manufacturing techniques cannot adequately meet [,].
Additive manufacturing (AM), commonly known as 3D printing, has significantly advanced the production of bone scaffolds, providing critical solutions for regenerative medicine. Unlike traditional methods such as solvent casting, gas foaming, and freeze-drying, AM offers precise control over scaffold geometry, porosity, and complexity—essential for effective bone regeneration [,]. The evolution of AM techniques, from fused deposition modeling (FDM) and selective laser sintering (SLS) to stereolithography (SLA), has enabled the creation of highly interconnected porous structures that facilitate cell adhesion, proliferation, and subsequent tissue growth [-].
More recently, 4D printing, an extension of 3D printing, has introduced scaffolds capable of dynamic responses to environmental stimuli such as temperature, moisture, and pH [,,]. While traditional AM focuses predominantly on static constructs, 4D printing incorporates temporal changes, enhancing scaffold functionality and adaptability for complex regenerative scenarios. However, despite the promising potential, practical implementation and consistent reproducibility of dynamic scaffolds remain challenging [,].
The advantages of additive manufacturing in bone scaffold production are significant. Primarily, AM facilitates patient-specific customization directly from medical imaging data, ensuring accurate anatomical conformity, unlike traditional approaches, which often rely on generalized designs []. Moreover, AM allows precise manipulation of scaffold architecture, improving nutrient transport, cellular integration, and biomechanical compatibility []. AM's layer-by-layer deposition significantly reduces material waste compared to subtractive techniques, aligning closely with sustainability objectives in healthcare manufacturing []. Additionally, the multi-material printing capabilities inherent to AM enable the integration of different biomaterials, thus creating complex gradient scaffolds mimicking the heterogeneous composition of native bone [,].
Despite these advancements, significant challenges persist, particularly in precision, biocompatibility, and scalability. Achieving submicron accuracy necessary for delicate scaffold features continues to be technically demanding due to irregularities in material deposition and process limitations []. Additionally, the biocompatibility of AM-produced scaffolds often necessitates extensive post-processing to remove residual toxicity from printing by-products, posing potential risks in clinical applications [,]. Furthermore, scalability and cost remain critical barriers, as AM is relatively slow and financially prohibitive for large-scale or batch production, limiting its adoption in resource-constrained healthcare settings [,].
Artificial intelligence (AI) has emerged as a transformative force within healthcare and manufacturing sectors, significantly influencing bone scaffold design and production through enhanced optimization processes, material selection, and automated quality control systems [,]. AI-driven generative design approaches rapidly optimize scaffold architectures, effectively replacing traditional trial-and-error methods that are often expensive and time-consuming []. For example, Pancholi, et al. [] demonstrated how generative algorithms successfully generated scaffold structures exhibiting improved porosity and mechanical strength compared to conventional designs.
In contrast, while LeCun, et al. (2015) highlight the effectiveness of deep learning for predictive modeling in scaffold design, Basu, et al. [] argue that the computational intensity and the data quality required for reliable predictions may limit practical feasibility. AI significantly contributes to material selection by evaluating extensive datasets to identify combinations that closely replicate natural bone properties, enhancing biodegradability, bioactivity, and mechanical robustness []. Yet, contrasting perspectives highlight the challenges in obtaining accurate, comprehensive databases essential for effective material prediction [].
Moreover, AI applications extend into manufacturing processes through real-time defect detection and quality assurance. Banerjee, et al [] illustrated AI's potential for monitoring AM processes, significantly reducing defect rates and material wastage. Conversely, Kumar, et al. [] stress the challenge of integrating complex AI systems into existing clinical workflows, suggesting that practical adoption remains slow due to cost and training constraints. Figure 2 summarizes the background and current challenges faced in the bone scaffold manufacturing process.
![Conceptual map of current challenges in scaffold design and production, including material, biological, and technological barriers. Adapted from []](https://www.igminresearch.com/articles/figures/igmin316/igmin316.g002.png) Figure 2: Conceptual map of current challenges in scaffold design and production, including material, biological, and technological barriers. Adapted from [,,]
Figure 2: Conceptual map of current challenges in scaffold design and production, including material, biological, and technological barriers. Adapted from [,,]Regarding sustainable manufacturing practices, AI's capability to optimize energy usage and material efficiency is increasingly critical. Verma, et al. [] demonstrated AI's effectiveness in reducing the environmental footprint of scaffold production through energy optimization and facilitating recycling and reuse initiatives, thereby supporting circular economy principles. Mittal, et al. [,] caution against potential socioeconomic disparities, suggesting that high-tech AI solutions might disproportionately benefit resource-rich settings despite these advances.
Therefore, while AI holds substantial promise for enhancing bone scaffold production and sustainability, addressing precision, cost-effectiveness, and equitable access challenges remains vital for broader implementation in healthcare settings.
In summary, conventional methods and materials provide only partial solutions for scaffold design. Polymers, ceramics, and composites each have strengths but also significant weaknesses, particularly when fabricated with traditional methods. Additive manufacturing addresses precision and customization, while AI promises predictive power and optimization. Together, they set the stage for a new generation of intelligent, patient-specific scaffolds.
The combination of artificial intelligence (AI) and additive manufacturing (AM) has significantly transformed the design and development of bone scaffolds. Researchers can create highly optimized scaffold structures tailored to each patient's needs by integrating AI. This has shifted scaffold design from traditional, manual methods towards innovative, automated approaches that use AI to predict and enhance scaffold performance.
Generative design uses AI algorithms to automatically generate scaffold structures based on specific requirements, including strength, porosity, and biocompatibility. Unlike traditional design methods, which often rely on manual trial-and-error approaches, AI-driven generative design rapidly evaluates numerous potential solutions, significantly speeding up the design process [].
For example, AI can develop scaffold geometries uniquely tailored to the individual's bone defect using patient-specific imaging data such as CT or MRI scans. These scaffold structures can mimic natural bone by having precisely controlled porous networks that facilitate cell growth and nutrient transport. AI progressively improves scaffold structures through iterative learning from simulations and previous designs, ensuring each subsequent version is more effective and reliable [].
Topology optimization represents another critical advancement, where AI algorithms reduce unnecessary material while maintaining the scaffold's structural integrity. This method is particularly beneficial for creating lightweight but strong scaffolds suitable for load-bearing applications like spinal implants or extensive bone repairs [].
AI also predicts scaffold performance before production, enabling detailed simulations of scaffolds' behavior under real-world conditions. These advanced AI-driven simulations assess critical properties such as mechanical stability, elasticity, and degradation rate, eliminating the need for extensive physical testing [].
AI simulations are beneficial for determining the optimal balance between scaffold porosity and mechanical strength. While porosity is necessary for tissue growth and nutrient exchange, excessive porosity may weaken structural integrity. AI helps designers navigate this balance efficiently, optimizing scaffold designs for maximum effectiveness [].
Moreover, AI can predict biocompatibility, helping researchers evaluate how different materials interact with biological environments. Machine learning models analyze historical data to predict potential inflammatory or immune responses, reducing risks associated with implant materials and accelerating material selection processes [].
Incorporating AI into additive manufacturing processes has revolutionized how bone scaffolds and other biomedical products are produced. AI technologies now play a critical role in process monitoring, quality control, and overall manufacturing optimization, addressing key challenges such as defect prevention, waste reduction, and sustainability.
One significant challenge in AM is the risk of manufacturing defects, such as voids, delamination, or inconsistent layering, which can compromise scaffold integrity and biocompatibility. AI addresses these issues through advanced defect detection systems that utilize real-time monitoring and predictive analytics.
For example, AI-powered computer vision systems capture high-resolution images during the printing process, analyzing these images with convolutional neural networks (CNNs) to rapidly detect potential defects such as uneven deposition or poor layer bonding []. Early detection allows immediate corrective actions, significantly reducing waste and ensuring higher quality.
AI also integrates sensor data— temperature, pressure, and laser intensity—to predict possible failures. By training on historical production data, AI models can proactively adjust parameters, enhancing consistency and precision in scaffold production [].
AI substantially contributes to reducing material waste and promoting sustainability in additive manufacturing. Due to their subtractive nature, traditional manufacturing processes frequently result in substantial material loss. In contrast, AI-driven additive manufacturing optimizes material usage, significantly minimizing waste. The benefits of integrating AI and AM are highlighted in Figure 3, which provides a radar analysis of key performance metrics.
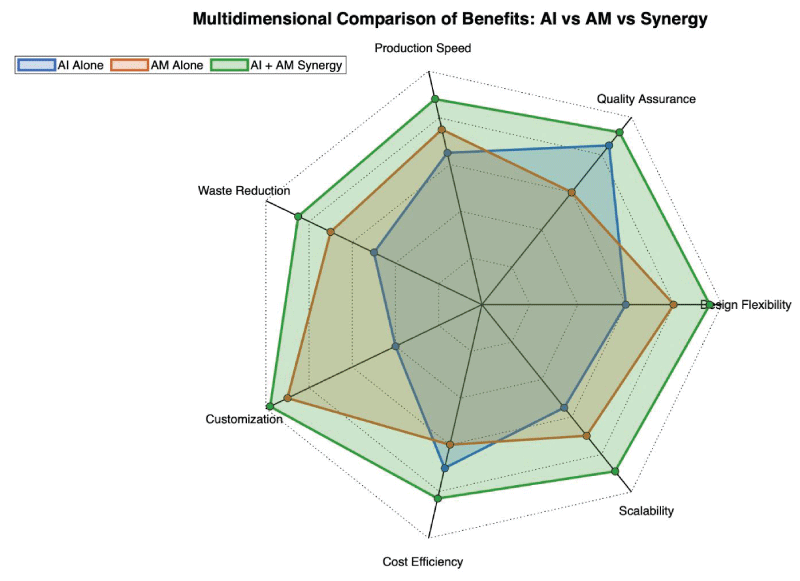 Figure 3: Comparative radar plot showing the benefits of AI alone, AM alone, and their integration. AI-AM integration offers superior material efficiency, sustainability, and scalability. Original work by the author.
Figure 3: Comparative radar plot showing the benefits of AI alone, AM alone, and their integration. AI-AM integration offers superior material efficiency, sustainability, and scalability. Original work by the author.AI analyzes manufacturing data to suggest improvements in energy efficiency and material consumption. Generative design powered by AI not only refines scaffold geometry but also ensures minimal use of valuable biomaterials such as polyetheretherketone (PEEK) or hydroxyapatite (HAP) while maintaining structural performance [].
Further, AI systems enhance sustainability through efficient material recycling. Machine learning can classify and repurpose residual printing materials for subsequent manufacturing cycles, greatly reducing waste []. AI-driven optimization also extends to energy usage, reducing the overall environmental impact by optimizing printer paths, energy settings, and production efficiency [].
Across the literature, a clear trend is visible. AI-driven generative design consistently reduces the need for trial-and-error in scaffold development and often improves compressive strength and porosity control by around twenty to thirty percent compared with traditional methods [,]. Yuan and colleagues showed that lattice structures designed with AI achieved more uniform stress distribution [], while Zarei and collaborators demonstrated accelerated healing with gradient porous scaffolds []. These findings highlight the strong potential of AI in scaffold optimization. At the same time, reproducibility between laboratories remains inconsistent, suggesting that standardized design software and shared datasets will be essential for wider clinical acceptance [].
Most of the studies employed established tools to support their results. Finite element analysis with software such as ANSYS, ABAQUS, and COMSOL was commonly used to predict compressive strength and elasticity [], []. Imaging methods like micro-CT and scanning electron microscopy validated pore architecture [,]. Machine learning approaches typically rely on Python libraries such as TensorFlow and PyTorch, enabling predictive modeling of material properties []. The technical sophistication of these methods is evident, but few reports comment on the computational cost of the AI models or validate their predictions through long-term in vivo studies. This gap must be addressed if these approaches are to translate into reliable clinical practice [,].
The synergy between AI and AM has led to significant advancements in scaffold design and patient-specific implants, demonstrating remarkable potential in addressing complex bone regeneration challenges.
AI-driven algorithms have successfully produced highly optimized bone scaffolds, carefully balancing mechanical properties, porosity, and bioactivity. Yuan, et al. [] used generative AI to design scaffolds featuring intricate lattice structures optimized for load-bearing applications. AI-guided simulations predicted optimal stress distribution, resulting in scaffolds with superior mechanical properties and better biological integration than traditional designs.
Similarly, Zarei, et al. [] employed AI to design gradient porous scaffolds to repair large bone defects. A conceptual framework for integrating AI and AM in clinical applications is shown in Figure 4. Their AI approach ensured consistent pore sizes and distributions, facilitating efficient nutrient flow and cell proliferation, with clinical studies showing substantial bone regeneration within eight weeks.
![Framework demonstrating how AI and AM can be integrated for clinical applications of bone scaffolds. Patient-specific imaging data flow into AI-driven design, which is then fabricated by AM and validated in vivo. Adapted from [].](https://www.igminresearch.com/articles/figures/igmin316/igmin316.g004.png) Figure 4: Framework demonstrating how AI and AM can be integrated for clinical applications of bone scaffolds. Patient-specific imaging data flow into AI-driven design, which is then fabricated by AM and validated in vivo. Adapted from [-].
Figure 4: Framework demonstrating how AI and AM can be integrated for clinical applications of bone scaffolds. Patient-specific imaging data flow into AI-driven design, which is then fabricated by AM and validated in vivo. Adapted from [-].AI has further enabled complex multi-material scaffold designs. Omigbodun, et al. [] utilized AI to precisely optimize the ratio of hydroxyapatite (HAp) and polylactic acid (PLA), creating scaffolds that exhibited controlled degradation synchronized with new bone formation.
AI-AM integration has significantly advanced patient-specific implants, improving patient outcomes by precisely matching individual anatomical features. For example, Verma, et al. [] employed AI-driven analysis of 3D imaging data to create customized cranial implants for traumatic brain injury patients. These implants precisely matched patients' skull contours, reducing surgical time and enhancing recovery outcomes.
Additionally, Monfred, et al. [] demonstrated the effectiveness of AI-designed mandibular implants, significantly improving functional outcomes for patients following cancer-related surgeries. AI-enhanced orthopedic implants, such as spinal cages designed by Mahmood, et al. [], showed optimized porosity for improved bone integration, enhancing mobility and reducing patient pain post-implantation.
Bone is a dynamic tissue regulated by osteoblasts, osteoclasts, and the extracellular matrix (ECM). Vascularization and mineral deposition are critical for the successful integration of scaffolds. Scaffold design must therefore support cell adhesion, angiogenesis, and gradual load transfer []. Current AI-driven approaches rarely model these complex biological pathways. Including parameters such as immune response and protein adsorption into AI training datasets could improve prediction accuracy.
Without integrating biological and biochemical realities, scaffold design remains incomplete. AI-based models need refinement to capture these factors.
Bone regeneration is governed not only by mechanical stability but also by cellular and biochemical processes. Osteoblasts and osteoclasts regulate new tissue growth, while angiogenesis ensures that nutrients and oxygen reach regenerating regions [,]. Growth factors such as BMP-2 and VEGF are particularly important in guiding these processes []. Scaffold design must therefore support both mechanical integrity and biological signaling. Yet most AI-driven approaches concentrate almost exclusively on geometry and mechanics, overlooking these biochemical requirements. Incorporating biological datasets into predictive models would allow AI to design scaffolds that are not only structurally sound but also biologically responsive.
Overall, AI brings predictive design and optimization, while AM ensures accurate, customized fabrication. When combined, they provide unique advantages in scaffold personalization, defect detection, and sustainability. However, achieving clinical translation requires addressing reproducibility, scalability, and long-term biological performance. These considerations lead directly into the broader discussion of sustainability, ethics, and future opportunities.
Integrating artificial intelligence (AI) with additive manufacturing (AM) significantly advances sustainability in biomedical manufacturing, especially in producing bone scaffolds. This synergy addresses critical environmental issues such as reducing material waste, optimizing energy use, and minimizing ecological footprints compared to traditional manufacturing methods.
Traditional manufacturing methods, including subtractive processes like milling or molding, inherently produce substantial material waste. In contrast, AM builds objects layer by layer, inherently minimizing waste. AI integration further enhances this efficiency by optimizing scaffold designs and refining the production process to utilize minimal materials while maintaining structural integrity [,].
AI-driven generative design algorithms can optimize structures by analyzing complex parameters like mechanical loading conditions, scaffold geometry, and porosity. Xin, et al. [] reported that AI-optimized bone scaffold designs reduce material consumption by approximately 30%, thus significantly lowering waste compared to conventional AM processes without AI optimization. Conversely, traditional AM methods without AI often rely on generic or manually derived structures, resulting in less efficient use of materials [].
Moreover, AI-powered defect detection systems greatly enhance material efficiency by identifying and addressing anomalies during the printing process. Li, et al. [] demonstrated that real-time defect detection significantly reduces the number of defective scaffolds produced, thereby conserving materials and energy. Without AI, defect identification typically occurs post-production, leading to higher waste and reduced sustainability.
AM's environmental sustainability also heavily relies on material selection. AI supports selecting biodegradable and environmentally friendly materials such as polylactic acid (PLA) and hydroxyapatite (HAp). AI algorithms evaluate extensive databases to optimize combinations of biodegradable polymers and bioactive ceramics, improving clinical performance and sustainability [].
Further, AI facilitates the integration of recycled and renewable materials into AM. Mohammadnabi, et al. [] successfully demonstrated using recycled PLA in scaffold production, achieving similar mechanical and biological performance compared to new materials. This supports the circular economy by effectively utilizing post-consumer waste, significantly lowering environmental impacts compared to traditional single-use manufacturing practices.
Additive manufacturing processes, particularly those involving biomedical scaffolds, are energy-intensive. AI integration offers significant potential to enhance energy efficiency and reduce the carbon footprint associated with these processes.
AI optimizes energy usage in AM by dynamically analyzing production data and adjusting parameters such as printing speed, layer thickness, and laser intensity. Studies indicate AI-driven optimization can substantially reduce energy consumption. For instance, Cong, et al. [] found that AI-enhanced AM processes decreased energy usage by approximately 25% by fine-tuning laser intensity and printing speeds in real-time.
Furthermore, AI-driven analyses of toolpaths minimize unnecessary movements, reducing energy use significantly, especially in large-scale, complex scaffolds. Such efficiency contrasts sharply with traditional AM, which lacks dynamic, real-time adjustments and often consumes excessive energy due to less optimized parameters [].
Lifecycle analysis (LCA) evaluates the comprehensive environmental impact of products throughout their entire lifecycle. AI significantly enhances LCA by providing detailed assessments and optimization at each stage:
Material Selection: AI systems identify sustainable and biodegradable materials suitable for scaffold production, thus reducing environmental impacts from raw material sourcing [].
Manufacturing Process Optimization: AI continuously optimizes production parameters, significantly reducing emissions and resource use compared to conventional methods [].
Recycling and Reuse: AI facilitates recycling efforts by identifying and sorting reusable materials efficiently, promoting waste reduction and circular economy principles [].
Carbon Footprint Tracking: AI-enabled LCA tools systematically track and analyze carbon emissions at each production phase, guiding strategic improvements and lowering overall emissions [].
Important uncertainties remain in our understanding of bone physiology as it relates to scaffold performance. Bone remodeling is influenced by age, hormonal balance, and metabolic health, and it varies across patient populations [,]. Most predictive models assume a uniform biological response, which does not reflect reality. Immune reactions such as inflammation and foreign body responses are also underexplored in computational models, even though they play a decisive role in the success or failure of an implant []. Recognizing these uncertainties prevents overgeneralization and underscores the need for interdisciplinary data that capture mechanical, biological, and clinical variables together [].
Despite the clear environmental and clinical advantages, integrating AI and AM introduces significant challenges and ethical considerations, particularly regarding sustainability and equitable healthcare access. Figure 5 visualizes the overlapping and distinct challenges faced in AI, additive manufacturing, and their combined use through a parallel coordinates plot.
![Parallel coordinates plot visualizing overlapping and unique challenges in AI, AM, and their combined use, including sustainability, equity, and scalability concerns [].](https://www.igminresearch.com/articles/figures/igmin316/igmin316.g005.png) Figure 5: Parallel coordinates plot visualizing overlapping and unique challenges in AI, AM, and their combined use, including sustainability, equity, and scalability concerns [-].
Figure 5: Parallel coordinates plot visualizing overlapping and unique challenges in AI, AM, and their combined use, including sustainability, equity, and scalability concerns [-].The extensive computational resources and energy requirements for training advanced AI models pose sustainability concerns. High-performance computing for AI model training generates substantial carbon emissions, comparable to the lifetime emissions of multiple vehicles []. Zhou, et al. [] highlighted that the energy used for AI training could constitute up to 15% of the total environmental impact of AM operations.
Mitigating these impacts requires exploring energy-efficient AI training methods, such as transfer learning, smaller specialized models, and the use of renewable energy sources for computing infrastructures []. Transparent reporting of AI models' environmental impacts is critical to drive accountability and improve sustainability.
While AI-driven AM offers significant benefits, the high costs associated with advanced technologies can exacerbate healthcare inequalities, especially in developing regions. Patient-specific, AI-designed implants typically entail higher costs, potentially limiting access to affluent patient populations or well-funded healthcare systems [,].
To address these ethical concerns, there is a growing emphasis on developing affordable, accessible solutions through open-source platforms, low-cost materials, and collaborative partnerships involving governments, private entities, and non-profits. Equitable healthcare access requires deliberate policy frameworks and industry practices that emphasize inclusivity and affordability. Sustainability metrics comparing traditional and AI-enhanced AM are summarized in Table 1.
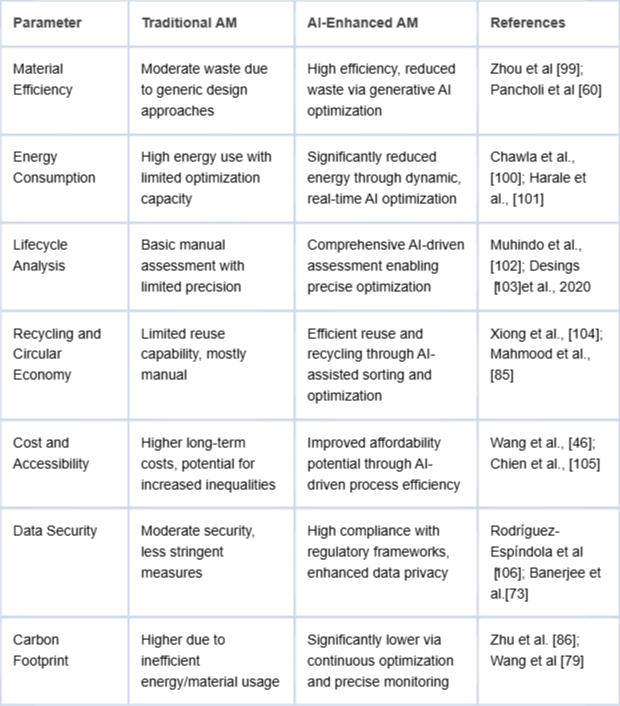 Table 1: Comparative analysis of sustainability indicators between traditional AM and AI-enhanced AM. Includes metrics on energy, waste, lifecycle, and cost.
Table 1: Comparative analysis of sustainability indicators between traditional AM and AI-enhanced AM. Includes metrics on energy, waste, lifecycle, and cost.The dependence of AI-driven healthcare solutions on patient data introduces critical data privacy concerns. Compliance with stringent data privacy regulations such as HIPAA and GDPR is essential to ensure patient confidentiality and secure data handling. Adequate data security protocols and transparency in data use practices are paramount to maintain patient trust and ensure broad acceptance of AI-enhanced healthcare innovations.
Despite promising outcomes, gaps remain. AI models are rarely validated against long-term clinical data, limiting reliability. Scaffold degradation and bone remodeling vary significantly between patients, yet models often assume uniform biological responses. Furthermore, the immune system’s role is underexplored, creating uncertainty in predicting implant performance.
The benefits of AI in scaffold design are compelling. It shortens design cycles, personalizes implants, and improves quality control during manufacturing [,]. At the same time, the risks must be acknowledged. Many algorithms operate as black boxes, limiting clinical transparency []. If training datasets are not representative, biases can emerge and affect patient outcomes []. The cost of implementing AI and advanced manufacturing could also deepen the divide between well-resourced healthcare systems and those in developing regions []. Patient data privacy is another pressing concern, since these systems rely heavily on sensitive imaging and clinical records []. Presenting these risks alongside the benefits encourages readers and practitioners to interpret results with caution and to approach adoption responsibly.
The ongoing advancement in materials and technologies promises revolutionary transformations in bone scaffold production. Innovations in smart biomaterials and the integration of artificial intelligence (AI) and the Internet of Things (IoT) are poised to significantly enhance regenerative medicine and personalized healthcare. These advancements will greatly improve scaffold functionality, patient outcomes, and implant monitoring capabilities, ushering in a new era of healthcare delivery.
Smart biomaterials represent a major innovation frontier, offering dynamic properties such as self-healing, shape memory, controlled drug delivery, and responsiveness to external stimuli. These advanced capabilities enable precise control over the scaffold's therapeutic actions and interactions within the body.
Shape-memory polymers, for instance, provide significant clinical advantages by facilitating minimally invasive implantation procedures. Scaffolds can be temporarily compressed into compact forms for easier surgical insertion and subsequently recover their optimal shape upon exposure to physiological stimuli such as temperature or pH changes []. Additionally, stimuli-responsive hydrogels offer targeted therapeutic delivery by releasing growth factors, antibiotics, or other bioactive agents in response to environmental triggers, thereby accelerating healing and reducing infection risks [].
AI has further accelerated biomaterial innovation by systematically analyzing extensive datasets of materials and their biological interactions, predicting optimal compositions for enhanced clinical efficacy. Machine learning-driven material discovery processes significantly reduce experimentation time and resources, promoting rapid and precise development of next-generation biomaterials tailored for complex clinical scenarios [,].
The convergence of IoT and AI in medical implants is revolutionizing implant monitoring and patient management. Smart implants equipped with sensors for real-time tracking of mechanical stresses, biochemical changes, and tissue regeneration provide unprecedented clinical insights. AI algorithms analyze sensor-generated data, predict potential complications, and proactively recommend therapeutic adjustments.
For example, IoT-enabled smart scaffolds incorporating piezoelectric sensors can continuously monitor mechanical load, facilitating early detection of structural weakening or scaffold failure. Clinicians can then promptly intervene, adjusting treatments to prevent clinical complications and enhance patient outcomes []. Furthermore, AI-driven predictive analytics enable personalized therapeutic strategies such as precise timing of controlled drug delivery, significantly optimizing patient recovery trajectories [,].
While AI and additive manufacturing (AM) technologies promise transformative healthcare advancements, scaling these innovations broadly faces critical economic and logistical challenges. Addressing these barriers is essential to unlock substantial global health improvements, particularly in resource-limited environments.
Significant upfront investments associated with high-performance 3D printers, proprietary software, and advanced biomaterials often hinder broad adoption. To mitigate these financial constraints, open-source AI platforms and modular AM systems are emerging as strategic solutions. Open-source frameworks, such as TensorFlow and PyTorch, substantially reduce software costs and facilitate collaborative innovation, democratizing technology access [,].
Modular AM systems offer economic advantages by enabling incremental upgrades of specific components rather than full system replacements, substantially reducing lifecycle costs. Concurrent advancements in material science further drive affordability by promoting the use of sustainable, locally sourced, and recycled biomaterials [,].
Public-private partnerships, governmental subsidies, and international funding initiatives are pivotal in reducing adoption barriers, incentivizing healthcare institutions to integrate these advanced technologies effectively [].
The strategic deployment of AI-AM technologies can address significant healthcare disparities in resource-limited settings. Portable and localized 3D printing systems can rapidly produce patient-specific scaffolds onsite, eliminating logistical and infrastructural barriers associated with imported medical products. AI-driven resource optimization further enables efficient material utilization, making scaffold production economically viable even in low-resource contexts [,].
Additionally, semi-automated AI-driven production methods reduce the dependency on highly specialized personnel, empowering local healthcare providers to deliver advanced treatments with minimal training. This democratization of healthcare technology significantly enhances global healthcare accessibility and quality [,].
Sustainability is becoming a critical focus for healthcare systems worldwide, with AI and AM positioned as key enablers of environmentally responsible healthcare solutions through circular economy models and enhanced medical waste recycling processes.
AI-driven circular economies within medical manufacturing optimize resource efficiency and reduce waste through predictive analytics and advanced generative designs. Automated AI systems streamline the recovery and recycling of biomaterials like PLA and hydroxyapatite, significantly reducing waste and promoting sustainable manufacturing practices [,].
Predictive maintenance informed by AI extends the operational lifespan of medical devices, decreasing the environmental impact by reducing the demand for new materials and mitigating waste generation. Emerging trends and innovations in scaffold production are detailed in Table 2. AI-guided generative design further minimizes material usage in scaffold production, aligning closely with sustainable manufacturing principles [,].
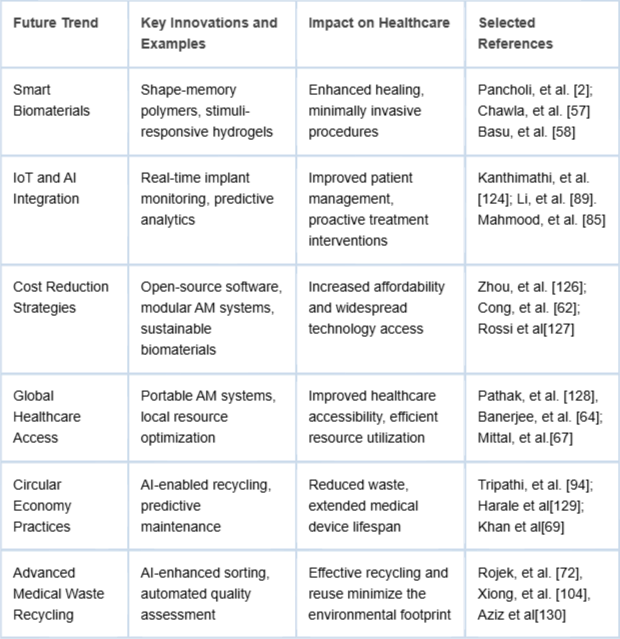 Table 2: Future trends in AI-AM scaffold design with innovations, expected impact, and example studies.
Table 2: Future trends in AI-AM scaffold design with innovations, expected impact, and example studies.Medical waste management is increasingly critical due to rising healthcare demands and the proliferation of disposable medical products. AI-enhanced recycling technologies significantly improve sorting efficiency and recovery rates of reusable medical materials, such as biopolymers and metallic alloys. Advanced AI algorithms automate the segregation and quality assessment processes, ensuring the recycled materials' suitability for reuse in subsequent medical applications [,].
AI-driven closed-loop manufacturing systems meticulously track resource use throughout production, usage, and recycling stages, enabling continuous improvement in sustainability practices. For instance, Wang et al. [] demonstrated a substantial reduction in medical waste by integrating AI-enabled recycling systems into scaffold production, significantly decreasing material loss and improving manufacturing efficiency.
While AI enhances personalization and efficiency, it also carries risks. Over-reliance on “black-box” models could obscure errors, and biases in training datasets may lead to unequal outcomes. High implementation costs risk widening disparities between high- and low-resource settings. Regulatory frameworks are still evolving, leaving uncertainties in clinical adoption.
AI should be seen as a powerful aid, not a replacement for clinical judgment. A cautious, transparent approach is essential.
Hydroxyapatite coatings remain one of the most effective strategies for enhancing the bioactivity of polymeric and metallic implants. PEEK implants coated with hydroxyapatite have shown stronger cell adhesion and improved osseointegration [,], although the long-term stability of the coating under physiological loading is still a concern. Reinforced scaffolds, such as PLA combined with hydroxyapatite, generally display higher compressive strength and more controlled degradation than single-material scaffolds [-]. Studies often report improvements of twenty to forty percent in mechanical properties, although performance varies depending on how well the two phases bond. Recent work has used AI to predict coating adhesion and optimize composite ratios [], which offers promising ways to overcome these challenges. Including coated and reinforced scaffolds within the AI-AM framework adds breadth and practical relevance to this review.
The future of AI and AM in scaffold design lies in smart biomaterials, IoT-enabled monitoring, cost reduction strategies, and circular economy practices. These advances have the potential to democratize access to regenerative medicine worldwide. However, realizing these opportunities depends on solving current gaps in biological integration, reproducibility, and regulatory oversight. This sets the foundation for a balanced conclusion, emphasizing both promise and caution.
The integration of artificial intelligence (AI) and additive manufacturing (AM) represents a significant leap forward in the design, development, and implementation of advanced bone scaffolds. This comprehensive review has systematically discussed the critical advancements, persistent challenges, and potential transformative impacts these technologies hold for healthcare, environmental sustainability, and global accessibility. Strategic recommendations for AI-AM healthcare integration are provided in Table 3.
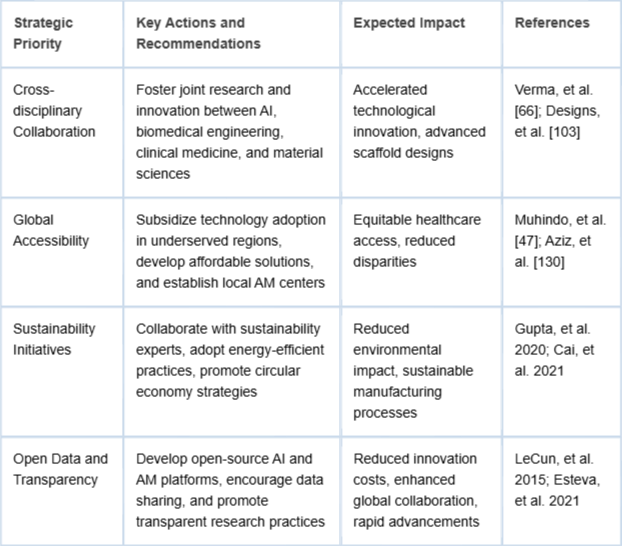 Table 3: Strategic recommendations for advancing AI-AM in healthcare, including collaboration, accessibility, sustainability, and transparency.
Table 3: Strategic recommendations for advancing AI-AM in healthcare, including collaboration, accessibility, sustainability, and transparency.AI has revolutionized scaffold design through generative modeling, predictive simulations, and sophisticated optimization techniques. These advancements have substantially improved scaffold architectures, enabling tailored mechanical properties, optimal porosity, enhanced biocompatibility, and patient-specific designs. Simultaneously, additive manufacturing technologies have made it possible to fabricate complex, patient-customized scaffolds precisely and sustainably, significantly reducing waste compared to traditional manufacturing methods.
The key breakthroughs highlighted throughout this review include:
AI-driven design optimization: Advanced generative design algorithms and deep learning approaches allow the creation of scaffolds optimized for individual patient requirements, significantly reducing material use and improving clinical performance.
Smart biomaterials and IoT integration: Innovations such as stimuli-responsive materials, smart hydrogels, and IoT-enabled implants have provided dynamic functionality and real-time monitoring capabilities, greatly enhancing the therapeutic potential and management of bone scaffolds.
Sustainability advancements: Integrating AI into manufacturing processes has considerably improved energy efficiency, minimized material wastage, and facilitated the effective recycling and reuse of medical biomaterials. These initiatives contribute substantially to developing a robust circular economy in medical manufacturing.
However, several critical challenges must be overcome to fully realize the potential of these technologies, including the high implementation costs, substantial energy demands associated with AI systems, and accessibility issues in resource-constrained regions.
The successful adoption and broad implementation of AI and AM technologies require a strategic, interdisciplinary, collaborative effort involving researchers, clinicians, engineers, policymakers, industry leaders, and sustainability experts. Effective collaboration across these domains will enable the resolution of critical challenges, accelerate innovation, and promote widespread access to advanced medical technologies. Essential actions include:
Promoting cross-disciplinary research collaborations: Engaging AI specialists, biomedical engineers, clinicians, and materials scientists to drive innovative research and develop advanced, next-generation scaffolds and implants.
Improving global accessibility: Implementing supportive governmental policies, financial incentives, public-private partnerships, and community-driven initiatives to facilitate widespread technology deployment in underserved regions, ensuring equitable access.
Advancing sustainability practices: Collaborating with environmental and sustainability experts to mitigate energy and resource challenges associated with AI and AM, ensuring alignment with global sustainability targets.
Encouraging open data and transparency: Developing and promoting open-source AI models, software platforms, and accessible material databases to accelerate innovation, reduce costs, and foster global collaboration.
The collective pursuit of these strategic priorities can significantly transform global healthcare delivery, enhance patient outcomes, promote sustainable practices, and ensure equitable access to innovative, personalized medical solutions. These technologies have immense potential to positively impact global health systems, environmental sustainability, and the democratization of advanced healthcare technologies.
Cong B, Zhang H. Innovative 3D printing technologies and advanced materials revolutionizing orthopedic surgery: current applications and future directions. Front Bioeng Biotechnol. 2025 Feb 11;13:1542179. doi: 10.3389/fbioe.2025.1542179. PMID: 40008034; PMCID: PMC11850356.
Pancholi S, Shaikh S, Kharbas S, Bhowmik S, Natarajan S, Khakhar D, et al. Transforming Additive Manufacturing with Artificial Intelligence: A Review of Current and Future Trends. Arch Comput Methods Eng. 2025. doi: 10.1007/S11831-025-10283-Y.
Ahalya K, Babu K-H. Revolutionizing biomedical engineering: Extrusion-based hydroxyapatite printing for scaffold construction: A review. Elsevier; 2024 [accessed 2025 Jun 18]. Available from: https://www.sciencedirect.com/science/article/pii/S2773207X24000885
Górski F. Computer Aided Design of 3D Printable Anatomically Shaped Medical Devices: Methodologies and Applications. 1st ed. CRC Press; 2025 Jan. doi: 10.1201/9781003386544/COMPUTER-AIDED-DESIGN-3D-PRINTABLE-ANATOMICALLY-SHAPED-MEDICAL-DEVICES-FILIP-GORSKI.
Helguero CG, Saldana SJ, Jimenez OC, DeFelipe M, Koutsou AD. Trabecular Scaffolds’ Mechanical Properties of Bone Reconstruction Using Biomimetic Implants. Procedia CIRP. 2017;65:121–6. doi: 10.1016/j.procir.2017.04.033.
Ambekar RS, Kandasubramanian B. Progress in the Advancement of Porous Biopolymer Scaffold: Tissue Engineering Application. Ind Eng Chem Res. 2019;58(16):6163–94. doi: 10.1021/acs.iecr.8b05334.
Zhou J, See CW, Sreenivasamurthy S, Zhu D. Customized Additive Manufacturing in Bone Scaffolds-The Gateway to Precise Bone Defect Treatment. Research (Wash D C). 2023 Oct 9;6:0239. doi: 10.34133/research.0239. PMID: 37818034; PMCID: PMC10561823.
Foroughi AH, Valeri C, Razavi MJ. A review of computational optimization of bone scaffold architecture: methods, challenges, and perspectives. Prog Biomed Eng. 2024 Nov 21;7(1). doi: 10.1088/2516-1091/ad879a. PMID: 39655853.
Zarei A, Faridmehr A. Synergizing additive manufacturing and machine learning for advanced hydroxyapatite scaffold design in bone regeneration. Springer; 2024 [accessed 2025 May 8]. Available from: https://link.springer.com/article/10.1007/s41779-024-01084-w
Pemmada R, Gowtham NH, Xia Y, Basu B, Thomas V. ML and AI approaches for design of tissue scaffolds. In: Thomas V, Basu B, editors. Artificial Intelligence in Tissue and Organ Regeneration. 1st ed. Elsevier; 2023. p. 29–56. doi: 10.1016/B978-0-443-18498-7.00008-9.
Katti DR, Katti KS, Gaikwad HK, Jaswandkar SV. Artificial intelligence in multiscale scaffolds for cancer organoids testbed. In: Thomas V, Basu B, editors. Artificial Intelligence in Tissue and Organ Regeneration. 1st ed. Elsevier; 2023. p. 193–218. doi: 10.1016/B978-0-443-18498-7.00005-3.
Heljak MK, Kijeńska-Gawrońska E, Swieszkowski W. Computational methods in modeling of scaffolds for neural tissue engineering. In: Pawar PV, editor. Biomaterials for Neural Tissue Engineering. 1st ed. Elsevier; 2023. p. 201–19. doi: 10.1016/B978-0-323-90554-1.00002-1.
Baino F, Ferraris S, Baino E, Vitale-Brovarone C. Recent trends in design, manufacturing and challenges of bone-like bioceramic scaffolds. Ceram Int. 2025 Apr. doi: 10.1016/J.CERAMINT.2025.02.307.
Ibrahimi S, D’Andrea L, Gastaldi D, Rivolta MW, Vena P. Machine learning approaches for the design of biomechanically compatible bone tissue engineering scaffolds. Comput Methods Appl Mech Eng. 2024 Apr;423:116842. doi: 10.1016/j.cma.2024.116842.
Ibrahimi S, D’Andrea L, Gastaldi D, Rivolta MW, Vena P. Machine learning approaches for the design of biomechanically compatible bone tissue engineering scaffolds [Internet]. Amsterdam: Elsevier; 2024 [cited 2025 May 8]. Available from: https://www.sciencedirect.com/science/article/pii/S0045782524000987
Yuan X, Zhu W, Yang Z, He N, Chen F, Han X, Zhou K. Recent advances in 3D printing of smart scaffolds for bone tissue engineering and regeneration. Adv Mater. 2024 Aug;36(34):e2403641. doi: 10.1002/adma.202403641. Epub 2024 Jun 28. PMID: 38861754.
Necolau M, Ionita M, Popa A. Poly(propylene fumarate) composite scaffolds for bone tissue engineering: Innovation in fabrication techniques and artificial intelligence integration [Internet]. Basel: MDPI; 2025 [cited 2025 May 8]. Available from: https://www.mdpi.com/2073-4360/17/9/1212
AI-enhanced bioactive 3D-printed scaffolds for tissue regeneration: Innovations in healing and functional additives [Internet]. J Comput Biomed Inform. [cited 2025 May 8]. Available from: https://jcbi.org/index.php/Main/article/view/863
Zhang ZZ, Jiang D, Ding JX, Wang SJ, Zhang L, Zhang JY, Qi YS, Chen XS, Yu JK. Role of scaffold mean pore size in meniscus regeneration. Acta Biomater. 2016 Oct 1;43:314-26. doi: 10.1016/j.actbio.2016.07.050. Epub 2016 Jul 29. PMID: 27481291.
Subia B, Kundu J, Choudhury S. Biomaterial scaffold fabrication techniques for potential tissue engineering applications. Tissue Eng. 2010 Jun; [Internet]. doi: 10.5772/8581.
Collins MN, Ren G, Young K, Pina S, Reis RL, Oliveira JM. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv Funct Mater. 2021 May;31(21):2010609. doi: 10.1002/adfm.202010609.
Mahanani ES, Puspita S, Dewi AH. The proper design of scaffold porosity for bone regeneration (literature review). AIP Conf Proc. 2024 May;3127(1):3294469. doi: 10.1063/5.0215991/3294469.
Korpela J, Kokkari A, Korhonen H, Malin M, Närhi T, Seppälä J. Biodegradable and bioactive porous scaffold structures prepared using fused deposition modeling. J Biomed Mater Res B Appl Biomater. 2013 May;101(4):610-9. doi: 10.1002/jbm.b.32863. Epub 2012 Dec 20. PMID: 23281260.
Janik and M. Marzec, ‘A review: Fabrication of porous polyurethane scaffolds’, Materials Science and Engineering C, vol. 48, pp. 586–591, 2015, doi: 10.1016/j.msec.2014.12.037. Janik H, Marzec M. A review: fabrication of porous polyurethane scaffolds. Mater Sci Eng C Mater Biol Appl. 2015 Mar;48:586-91. doi: 10.1016/j.msec.2014.12.037. Epub 2014 Dec 17. PMID: 25579961.
Chong ETJ, Ng JW, Lee PC. Classification and medical applications of biomaterials–a mini review. BIO Integration. 2023 Aug;4(2):54. doi: 10.15212/BIOI-2022-0009.
Oladapo BI, Zahedi SA, Chong S, Omigbodun FT, Malachi IO. 3D printing of surface characterisation and finite element analysis improvement of PEEK-HAP-GO in bone implant. 2019 [cited 2024 Oct 24]. Available from: https://dora.dmu.ac.uk/bitstream/2086/18915/3/3D%20printing%20of%20surface%20characterisation%20and%20finite%20element%20analysis%20improvement%20of%20tensile%20properties%20for%20PEEK-HAP-GO%20in%20bone%20implant.pdf
Oladapo BI, Zahedi SA, Chong S, Omigbodun FT, Malachi IO. 3D printing of PEEK–cHAp scaffold for medical bone implant. Biodes Manuf. 2021 Mar;4(1):44–59. doi: 10.1007/S42242-020-00098-0.
Zhao Y, Zhao K, Li Y, Chen F. Mechanical characterization of biocompatible PEEK by FDM. J Manuf Process. 2020 Apr;56:28–42. doi: 10.1016/j.jmapro.2020.04.063.
Olawumi MA, Omigbodun FT, Oladapo BI, Olugbade TO, Olawade DB. Innovative PEEK in dentistry of enhanced adhesion and sustainability through AI-driven surface treatments. Bioengineering (Basel). 2024 Sep 14;11(9):924. doi: 10.3390/bioengineering11090924. PMID: 39329666; PMCID: PMC11429295.
Oladapo BI, Ismail SO, Bowoto OK, Omigbodun FT, Olawumi MA, Muhammad MA. Lattice design and 3D-printing of PEEK with Ca10(OH)(PO4)3 and in-vitro bio-composite for bone implant. Int J Biol Macromol. 2020 Dec 15;165(Pt A):50–62. doi: 10.1016/j.ijbiomac.2020.09.175. PMID: 32979443.
Zhang L, Morsi Y, Wang Y, Li Y, Ramakrishna S. Review scaffold design and stem cells for tooth regeneration. Jpn Dent Sci Rev. 2013;49(1):14–26. doi: 10.1016/j.jdsr.2012.09.001.
Haghdan S, Smith GD. Natural fiber reinforced polyester composites: a literature review. 2015 Jul 11. doi: 10.1177/0731684415588938.
Siakeng R, Jawaid M, Ariffin H, Sapuan SM, Asim M, Saba N. Natural fiber reinforced polylactic acid composites: a review. Polym Compos. 2019;40(2):446–63. doi: 10.1002/pc.24747.
Omigbodun F. Manufacturing and mechanical characterization of PLA/cHAP/rGO composite for bone implants. 2024 Nov. doi: 10.26174/THESIS.LBORO.27867627.V1.
Omigbodun FT, Oladapo BI. Enhanced mechanical properties and degradation control of poly(lactic) acid/hydroxyapatite/reduced graphene oxide composites for advanced bone tissue engineering application. Biomimetics (Basel). 2024 Oct 23;9(11):651. doi: 10.3390/biomimetics9110651. PMID: 39590223; PMCID: PMC11592037.
Omigbodun FT, Oladapo BI, Osa-uwagboe N. Exploring the frontier of polylactic acid/hydroxyapatite composites in bone regeneration and their revolutionary biomedical applications – a review. J Reinf Plast Compos. 2024 Sep. doi: 10.1177/07316844241278045. Available from: https://journals.sagepub.com/doi/pdf/10.1177/07316844241278045
Omigbodun FT, Bowoto OK, Nimako PA, Elemure IE, Ojo GG. Reduction in economic cost and production time for development of a 3D printer and its effect on market economic. Int J Eng Trends Technol. 2019;67(6). doi: 10.14445/22315381/IJETT-V67I6P218.
Omigbodun F, Osa-Uwagboe N, Udu A, Oladapo BO. Leveraging machine learning for optimized mechanical properties and 3D printing of PLA/cHAP for bone implant. Biomimetics (Basel). 2024 [cited 2024 Oct 24]. Available from: https://www.mdpi.com/2313-7673/9/10/587
Oladapo BI, Zahedi SA, Omigbodun FT. A systematic review of polymer composite in biomedical engineering. Eur Polym J. 2021;154:110534. doi: 10.1016/j.eurpolymj.2021.110534.
Appuhamillage G, Ahmad SA, et al. 3D and 4D printing of biomedical materials: current trends, challenges, and future outlook. 2024 [cited 2025 Jun 18]. Available from: https://www.explorationpub.com/Journals/em/Article/1001203
Zhou J, See CW, Sreenivasamurthy S, Zhu D. Customized Additive Manufacturing in Bone Scaffolds-The Gateway to Precise Bone Defect Treatment. Research (Wash D C). 2023 Oct 9;6:0239. doi: 10.34133/research.0239. PMID: 37818034; PMCID: PMC10561823.
Kumar P, Rajak D, Abubakar M, Singh SA. 3D printing technology for biomedical practice: a review. J Mater Eng Perform. 2021. Available from: https://link.springer.com/article/10.1007/s11665-021-05792-3
Oladapo BI, Ismail SO, Omigbodun FT, Oluwole B, Olawumi MA, Samad YA. 4D Printing of Nanostructure Modified Shape Memory Polymer Composites. 2024. Available from: https://researchprofiles.herts.ac.uk/en/publications/4d-printing-of-nanostructure-modified-shape-memory-polymer-compos
Mehrpouya M, Vahabi H, Janbaz S, Darafsheh A, Mazur TR, Ramakrishna S. 4D printing of shape memory polylactic acid (PLA). Polymer (Guildf). 2021 Aug;230:124080. doi: 10.1016/j.polymer.2021.124080.
Ngo TD, Kashani A, Imbalzano G, Nguyen KTQ, Hui D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos B Eng. 2018 Feb;143:172–196. doi: 10.1016/j.compositesb.2018.02.012.
Wang DD, Qian Z, Vukicevic M, Engelhardt S, Kheradvar A, Zhang C, et al. 3D Printing, Computational Modeling, and Artificial Intelligence for Structural Heart Disease. JACC Cardiovasc Imaging. 2021 Jan;14(1):41-60. doi: 10.1016/j.jcmg.2019.12.022. Epub 2020 Aug 26. PMID: 32861647.
Muhindo D, Elkanayati R, Srinivasan P, Repka MA, Ashour EA. Recent Advances in the Applications of Additive Manufacturing (3D Printing) in Drug Delivery: A Comprehensive Review. AAPS PharmSciTech. 2023 Feb 9;24(2):57. doi: 10.1208/s12249-023-02524-9. Erratum in: AAPS PharmSciTech. 2023 Mar 10;24(3):75. doi: 10.1208/s12249-023-02542-7. PMID: 36759435.
Choi WJ, Hwang KS, Kwon HJ, Lee C, Kim CH, Kim TH, et al. Rapid development of dual porous poly(lactic acid) foam using fused deposition modeling (FDM) 3D printing for medical scaffold application. Mater Sci Eng C Mater Biol Appl. 2020 May;110:110693. doi: 10.1016/j.msec.2020.110693. Epub 2020 Jan 27. PMID: 32204007.
Ruiz-Alonso S, Lafuente-Merchan M, Ciriza J, Saenz-Del-Burgo L, Pedraz JL. Tendon tissue engineering: Cells, growth factors, scaffolds and production techniques. J Control Release. 2021 May 10;333:448-486. doi: 10.1016/j.jconrel.2021.03.040. Epub 2021 Mar 31. PMID: 33811983.
Turnbull G, Clarke J, Picard F, Riches P, Jia L, Han F, et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018 Sep;3(3):278–314. doi: 10.1016/j.bioactmat.2017.10.001.
Wang C, Wang M, Xu T, Zhang X, Lin K. 3D printing of bone tissue engineering scaffolds. Bioact Mater. 2020 Mar;5(1):82–91. doi: 10.1016/j.bioactmat.2020.01.004.
Farah S, Anderson DG, Langer R. Physical and mechanical properties of PLA, and their functions in widespread applications - A comprehensive review. Adv Drug Deliv Rev. 2016 Dec 15;107:367-392. doi: 10.1016/j.addr.2016.06.012. Epub 2016 Jun 26. PMID: 27356150.
Penumakala PK, Santo J, Thomas A. A critical review on the fused deposition modeling of thermoplastic polymer composites. Compos B Eng. 2020 Jul;201:108336. doi: 10.1016/j.compositesb.2020.108336.
Wang Y, Zhou Y, Lin L, Corker J, Fan M. Overview of 3D additive manufacturing (AM) and corresponding AM composites. Compos Part A Appl Sci Manuf. 2020 Jul;139:106114. doi: 10.1016/j.compositesa.2020.106114.
Almesmari A, Sheikh-Ahmad J, Jarrar F, Bojanampati S. Optimizing the specific mechanical properties of lattice structures fabricated by material extrusion additive manufacturing. J Mater Res Technol. 2023 Jan;22:1821–1838. doi: 10.1016/j.jmrt.2022.12.024.
Fedorenko A, Fedulov B, Jurgenson S, Lomakin E. 3D lattice-based structural elements for industrial application. Procedia Struct Integr. 2021;31:652–657. doi: 10.1016/j.prostr.2021.10.072.
Chawla T, Rukhaiyar V, Banu SA, Singh S. The future of tissue engineering: Integrating ML, AI, and computer vision. 2025. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003536352-2/future-tissue-engineering-tvisha-chawla-vaishnavi-rukhaiyar-shaik-arshid-banu-subhash-singh
Basu B, Gowtham N, Xiao Y, Kalidindi S, Lim K. Biomaterialomics: Data science-driven pathways to develop fourth-generation biomaterials. Acta Biomater. 2022. Available from: https://www.sciencedirect.com/science/article/pii/S1742706122001039
Rossi GD, Vergani L, Bonfanti F. A Novel Triad of Bio-Inspired Design, Digital Fabrication, and Bio-Derived Materials for Personalised Bone Repair. Materials (Basel). 2024. Available from: https://www.mdpi.com/1996-1944/17/21/5305
Pancholi S, Gupta M, Bansal M. Transforming Additive Manufacturing with Artificial Intelligence: A Review of Current and Future Trends. 2025. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003536352-2/future-tissue-engineering-tvisha-chawla-vaishnavi-rukhaiyar-shaik-arshid-banu-subhash-singh
Basu B, Gowtham N, Xiao Y, Kalidindi S. Biomaterialomics: Data science-driven pathways to develop fourth-generation biomaterials. Acta Biomater. 2022. Available from: https://www.sciencedirect.com/science/article/pii/S1742706122001039
Cong B, Zhao H. Innovative 3D printing technologies and advanced materials revolutionizing orthopedic surgery: current applications and future directions. Front Bioeng Biotechnol. 2025. Available from: https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1542179/full
Gorski F. Computer Aided Design of 3D Printable Anatomically Shaped Medical Devices: Methodologies and Applications. 2025. Available from: https://www.taylorfrancis.com/books/mono/10.1201/9781003386544/computer-aided-design-3d-printable-anatomically-shaped-medical-devices-filip-gorski
Banerjee A, Haridas H, Sen A. Artificial intelligence in 3D printing: a revolution in health care. In: Applications of 3D Printing in Healthcare. Springer; 2021. Available from: https://link.springer.com/chapter/10.1007/978-981-33-6703-6_4
Kumar N, Chakradhar D. Advancing bioimplant manufacturing through artificial intelligence. In: Bioimplants Manufacturing: Fundamentals and Advances. CRC Press; 2024. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003509943-13/advancing-bioimplant-manufacturing-artificial-intelligence-namadi-vinod-kumar-chakradhar-abhilash
Verma D, Dong Y, Sharma M, Saini M. Advanced processing of 3D printed biocomposite materials using artificial intelligence. Mater Manuf Process. 2022;37(5):518–538. Available from: https://www.tandfonline.com/doi/abs/10.1080/10426914.2021.1945090
Mittal R, Haleem A, Kumar A. Advances in additive manufacturing: artificial intelligence, nature-inspired, and biomanufacturing. 2022. Available from: https://books.google.com/books?hl=en&lr=&id=c8h6EAAAQBAJ&oi=fnd&pg=PP1
Monfred V. Application of Artificial Intelligence (Machine Learning) in Additive Manufacturing, Bio-Systems, Bio-Medicine, and Composites. In: Additive Manufacturing for Biocomposites and Synthetic Composites. CRC Press; 2023. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003362128-9
Khan SB, Irfan S, Zhang Z, Yuan W. Redefining Medical Applications with Safe and Sustainable 3D Printing. ACS Appl Bio Mater. 2025 Aug 18;8(8):6470–6525. doi: 10.1021/acsabm.4c01923. Epub 2025 Apr 8. PMID: 40200689. Available from: https://pubs.acs.org/doi/10.1021/acsabm.4c01923
Banerjee A, Haridas HK, SenGupta A, Jabalia N. Artificial Intelligence in 3D Printing: A Revolution in Health Care. In: Lecture Notes in Bioengineering. 2022:57–79. doi: 10.1007/978-981-33-6703-6_4. Available from: https://link.springer.com/chapter/10.1007/978-981-33-6703-6_4
Kumar NV, Chakradhar D, Abhilash PM. Advancing bioimplant manufacturing through artificial intelligence. In: Bioimplants Manufacturing: Fundamentals and Advances. 2024:284–312. doi: 10.1201/9781003509943-13. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003509943-13
Rojek I, Mikołajewski D, Dostatni E, Mazur M. AI-optimized technological aspects of the material used in 3D printing processes for selected medical applications. Materials (Basel). 2020;13(23):5437. Available from: https://www.mdpi.com/1996-1944/13/23/5437
Banerjee A, Haridas H, Sen A. Artificial intelligence in 3D printing: a revolution in health care. In: Applications of 3D Printing in Healthcare. Springer; 2021. Available from: https://link.springer.com/chapter/10.1007/978-981-33-6703-6_4
Wang Y, Zheng P, Peng T, Yang H, Zou J. Smart additive manufacturing: Current artificial intelligence-enabled methods and future perspectives. Sci China Technol Sci. 2020;63(9):1600–1611. Available from: https://link.springer.com/article/10.1007/s11431-020-1581-2
Yang J, Chen Y, Huang W, Liu Y. Survey on artificial intelligence for additive manufacturing. In: 2027 23rd International Conference on Document Analysis and Recognition (ICDAR). 2017. Available from: https://ieeexplore.ieee.org/abstract/document/8082053/
Goh G, Sing S, Yeong WY. A review on machine learning in 3D printing: applications, potential, and challenges. Artif Intell Rev. 2020;54(1):1–32. doi: 10.1007/s10462-020-09876-9. Available from: https://link.springer.com/article/10.1007/s10462-020-09876-9
Rodríguez-Espíndola O, Chowdhury S, Beltagui A, Albores P. The potential of emergent disruptive technologies for humanitarian supply chains: the integration of blockchain, Artificial Intelligence and 3D printing. Int J Prod Res. 2020;58(15):4610–4630. doi: 10.1080/00207543.2020.1761565. Available from: https://doi.org/10.1080/00207543.2020.1761565
Jin Z, Zhang Z, Guo G. Automated real‐time detection and prediction of interlayer imperfections in additive manufacturing processes using artificial intelligence. AI Systems. 2019;2(1). doi: 10.1002/aisy.201900130. Available from: https://onlinelibrary.wiley.com/doi/10.1002/aisy.201900130
Wang YB, Zheng P, Peng T, Yang HY, Zou J. Smart additive manufacturing: Current artificial intelligence-enabled methods and future perspectives. Sci China Technol Sci. 2020;63(9):1600–1611. doi: 10.1007/S11431-020-1581-2. Available from: https://link.springer.com/article/10.1007/s11431-020-1581-2
Yuan X, Zhu W, Yang Z, He N, Cheng F. Recent advances in 3D printing of smart scaffolds for bone tissue engineering and regeneration. Adv Mater. 2024. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.202403641
Zarei A, Farazin A. Synergizing additive manufacturing and machine learning for advanced hydroxyapatite scaffold design in bone regeneration. J Aust Ceram Soc. 2024. doi: 10.1007/S41779-024-01084-W. Available from: https://link.springer.com/article/10.1007/s41779-024-01084-w
Omigbodun FT, Oladapo BI. AI-Optimized Lattice Structures for Biomechanics Scaffold Design. Biomimetics (Basel). 2025 Feb 1;10(2):88. doi: 10.3390/biomimetics10020088. PMID: 39997111; PMCID: PMC11853473. Available from: https://www.mdpi.com/1996-1944/10/2/88
Verma D, Dong Y, Sharma M, Chaudhary AK. Advanced processing of 3D printed biocomposite materials using artificial intelligence. Mater Manuf Process. 2022;37(5):518–538. doi: 10.1080/10426914.2021.1945090. Available from: https://www.tandfonline.com/doi/abs/10.1080/10426914.2021.1945090
Monfred V. Application of artificial intelligence (machine learning) in additive manufacturing, bio-systems, bio-medicine, and composites. In: Additive Manufacturing for Biocomposites and Synthetic Composites. 2023:152–203. doi: 10.1201/9781003362128-9. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003362128-9
Mahmood M, Visan A, Ristoscu C, Mihailescu IN. Artificial neural network algorithms for 3D printing. Materials (Basel). 2020;14(1):163. Available from: https://www.mdpi.com/1996-1944/14/1/163
Zhu Z, Ng D, Park H, Materials MM-NR, et al. 3D-printed multifunctional materials enabled by artificial-intelligence-assisted fabrication technologies. Nat Rev Mater. 2021 [cited 2025 May 8]. Available from: https://www.nature.com/articles/s41578-020-00235-2
Xin H, Virk AS, Virk SS, Akin-Ige F, Amin S. Applications of artificial intelligence and machine learning on critical materials used in cosmetics and personal care formulation design. Curr Opin Colloid Interface Sci. 2024 Oct;73:101847. doi: 10.1016/j.cocis.2024.101847
Cong B, Zhang H. Innovative 3D printing technologies and advanced materials revolutionizing orthopedic surgery: current applications and future directions. Front Bioeng Biotechnol. 2025 Feb 11;13:1542179. doi: 10.3389/fbioe.2025.1542179. PMID: 40008034; PMCID: PMC11850356
Li Z, Song P, Li G, Han Y, Ren X, Bai L, Su J. AI energized hydrogel design, optimization and application in biomedicine. Mater Today Bio. 2024 Feb 29;25:101014. doi: 10.1016/j.mtbio.2024.101014. PMID: 38464497; PMCID: PMC10924066
Murali A, Parameswaran R. Extrusion 3D printing advances for craniomaxillofacial bone tissue engineering. Polym Plast Technol Mater. 2024;63(7):889–912. doi: 10.1080/25740881.2024.2307351
Mohammadnabi S, Moslemy N, Taghvaei H, Zia AW, Askarinejad S, Shalchy F. Role of artificial intelligence in data-centric additive manufacturing processes for biomedical applications. J Mech Behav Biomed Mater. 2025 Jun;166:106949. doi: 10.1016/j.jmbbm.2025.106949. Epub 2025 Feb 25. PMID: 40036906
Cong B, Zhang H. Innovative 3D printing technologies and advanced materials revolutionizing orthopedic surgery: current applications and future directions. Front Bioeng Biotechnol. 2025 Feb 11;13:1542179. doi: 10.3389/fbioe.2025.1542179. PMID: 40008034; PMCID: PMC11850356
Khan S, Irfan S, Zhang Z, Materials WYAAB, et al. Redefining medical applications with safe and sustainable 3D printing. ACS Appl Bio Mater. 2025 [cited 2025 Jun 18]. Available from: https://pubs.acs.org/doi/abs/10.1021/acsabm.4c01923
Tripathi S, Ansari A, Singh M, Dash M, P K-M, et al. Surgical planning and procedures through synergistic use of additive manufacturing, advanced materials and artificial intelligence: challenges and opportunities. Mater Horiz. 2025 [cited 2025 Jun 18]. Available from: https://pubs.rsc.org/en/content/articlelanding/2025/mh/d5mh00501a
Jeyaraman M, Nallakumarasamy A, Jeyaraman N. Industry 5.0 in orthopaedics. Indian J Orthop. 2022 Aug 23;56(10):1694–1702. doi: 10.1007/s43465-022-00712-6. PMID: 36187596; PMCID: PMC9485301
Ikhsan R, Rahayu S, R A-AJRI, et al. Integrating artificial intelligence with 3D printing technology in healthcare: sustainable solutions for clinical training optimization. Asian J Res Innov. 2025;6(2):99–107. doi: 10.34306/ajri.v6i2.1126
Zhou J, See C, Sreenivasamurthy S, Zhu D. Customized additive manufacturing in bone scaffolds—the gateway to precise bone defect treatment. Research. 2023 [cited 2025 Jun 18]. Available from: https://spj.science.org/doi/abs/10.34133/research.0239
Sharma G, Rawat K, S S-IE, et al. Adoption of controls and saga of development in 3D printed bioimplants: a business perspective. IEEE Eng. 2024 [cited 2025 Jun 18]. Available from: https://ieeexplore.ieee.org/abstract/document/10683896/
Zhou J, See CW, Sreenivasamurthy S, Zhu D. Customized additive manufacturing in bone scaffolds—The gateway to precise bone defect treatment. Research (Wash D C). 2023 Oct 9;6:0239. doi: 10.34133/research.0239. PMID: 37818034; PMCID: PMC10561823
Chawla T, Rukhaiyar V, Banu SA, Singh S, et al. The future of tissue engineering: integrating ML, AI, and computer vision. In: Advances in Tissue Engineering. Taylor & Francis; 2025 [cited 2025 Jun 18]. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003536352-2/future-tissue-engineering-tvisha-chawla-vaishnavi-rukhaiyar-shaik-arshid-banu-subhash-singh
Harale A, Jadhav D, Jadhav P, et al. Revolutionizing orthopedics through integration of artificial intelligence and 3D printing for enhanced patient care. Springer. 2025 [cited 2025 Jun 18]. Available from: https://link.springer.com/article/10.1007/s43465-025-01423-4
Muhindo D, Elkanayati R, Srinivasan P, et al. Recent advances in the applications of additive manufacturing (3D printing) in drug delivery: a comprehensive review. Springer. 2023 [cited 2025 May 8]. Available from: https://link.springer.com/article/10.1208/s12249-023-02524-9
J. Exploring the interrelationship between additive manufacturing and Industry 4.0. Designs. 2020 [cited 2025 May 8]. Available from: https://www.mdpi.com/2411-9660/4/2/13
Xiong Y, Tang Y, Zhou Q, Ma Y, Ren D. Intelligent additive manufacturing and design: state of the art and future perspectives. Addit Manuf. 2022 [cited 2025 May 8]. Available from: https://www.sciencedirect.com/science/article/pii/S2214860422005280
Chien C-F, Dauzère-Pérès S, Huh WT, Jang YJ, Morrison JR. Artificial intelligence in manufacturing and logistics systems: algorithms, applications, and case studies. Int J Prod Res. 2020 May;58(9):2730–1. doi: 10.1080/00207543.2020.1752488
Rodríguez-Espíndola O, Chowdhury S, Beltagui A, Albores P. The potential of emergent disruptive technologies for humanitarian supply chains: the integration of blockchain, artificial intelligence and 3D printing. Int J Prod Res. 2020 [cited 2025 May 8]. Available from: https://www.tandfonline.com/doi/abs/10.1080/00207543.2020.1761565
Murali A, Prabhu R. Extrusion 3D printing advances for craniomaxillofacial bone tissue engineering. Prog Addit Manuf. 2024 [cited 2025 May 8]. Available from: https://www.tandfonline.com/doi/abs/10.1080/25740881.2024.2307351
Zhou J, See C, Sreenivasamurthy S, Zhu D. Customized additive manufacturing in bone scaffolds—the gateway to precise bone defect treatment. Research. 2023 [cited 2025 May 8]. Available from: https://spj.science.org/doi/abs/10.34133/research.0239
Kolomenskaya E, Butova V, Poltavskiy A, Smirnov A. Application of artificial intelligence at all stages of bone tissue engineering. Biomedicines. 2023 [cited 2025 May 8]. Available from: https://www.mdpi.com/2227-9059/12/1/76
Eber P, Sillmann Y, Guastaldi FG. Beyond 3D printing: how AI is shaping the future of craniomaxillofacial bone tissue engineering. ACS Biomater Sci Eng. 2025 [cited 2025 May 8]. Available from: https://pubs.acs.org/doi/abs/10.1021/acsbiomaterials.5c00420
Eber P, Sillmann YM, Guastaldi FPS. Beyond 3D printing: how AI is shaping the future of craniomaxillofacial bone tissue engineering. ACS Biomater Sci Eng. 2025 Jun 9;11(6):3095–8. doi: 10.1021/acsbiomaterials.5c00420. Epub 2025 May 2. PMID: 40314572.
Ramachandran MK, Raigar J, Kannan M, Velu R. State-of-the-art overview and recent trends in biomedical devices using digital manufacturing: opportunities, limitations, and current market. In: Digital Design and Manufacturing of Medical Devices and Systems. 2023:1–31. doi: 10.1007/978-981-99-7100-8_1.
Kennedy SM, K A, JJB JJ, V E, RB JR. Transformative applications of additive manufacturing in biomedical engineering: bioprinting to surgical innovations. J Med Eng Technol. 2024 May;48(4):151–68. doi: 10.1080/03091902.2024.2399017. Epub 2024 Sep 16. PMID: 39282861.
Shokrani H, et al. Artificial intelligence for biomedical engineering of polysaccharides: a short overview. Curr Opin Biomed Eng. 2023 Sep;27:100463. doi: 10.1016/J.COBME.2023.100463.
Thomas V, Chandy T, Sharma CP. AI and ML: challenges and future perspective in artificial organs realm. In: Artificial Intelligence in Tissue and Organ Regeneration. 2023 Jan:303–16. doi: 10.1016/B978-0-443-18498-7.00015-6.
Wu C, et al. Topology optimisation for design and additive manufacturing of functionally graded lattice structures using derivative-aware machine learning algorithms. Addit Manuf. 2023 Sep;78:103833. doi: 10.1016/J.ADDMA.2023.103833.
Lin F, Basem A, Khaddour MH, Salahshour S, Li W, Sabetvand R. Advancing mechanical and biological characteristics of polymer-ceramic nanocomposite scaffolds for sport injuries and bone tissue engineering: a comprehensive investigation applying finite element analysis and artificial neural network. Ceram Int. 2025 Apr. doi: 10.1016/J.CERAMINT.2025.01.115.
Ramesh S, Deep A, Tamayol A, Kamaraj A, Mahajan C, Madihally S. Advancing 3D bioprinting through machine learning and artificial intelligence. Bioprinting. 2024 Apr;38:e00331. doi: 10.1016/J.BPRINT.2024.E00331.
Wu C, Wan B, Entezari A, Fang J, Xu Y, Li Q. Machine learning-based design for additive manufacturing in biomedical engineering. Int J Mech Sci. 2024 Mar;266:108828. doi: 10.1016/J.IJMECSCI.2023.108828.
Li Z, et al. Inverse design of cellular structures with the geometry of triply periodic minimal surfaces using generative artificial intelligence algorithms. Eng Struct. 2024 Dec;321:118988. doi: 10.1016/J.ENGSTRUCT.2024.118988.
Khan N, Asad H, Khan S, Riccio A. Towards defect-free lattice structures in additive manufacturing: a holistic review of machine learning advancements. J Manuf Process. 2025 Jun;144:1–53. doi: 10.1016/J.JMAPRO.2025.04.035.
Greenberg ZF, Graim KS, He M. Towards artificial intelligence-enabled extracellular vesicle precision drug delivery. Adv Drug Deliv Rev. 2023 Aug;199:114974. doi: 10.1016/j.addr.2023.114974. Epub 2023 Jun 23. PMID: 37356623.
Li W, Li L, Hu J, Zhou D, Su H. Design and Applications of Supramolecular Peptide Hydrogel as Artificial Extracellular Matrix. Biomacromolecules. 2024 Nov 11;25(11):6967-6986. doi: 10.1021/acs.biomac.4c00971. Epub 2024 Oct 17. PMID: 39418328.
Kanthimathi T, Rathika N, Fathima A. Robotic 3D Printing for Customized Industrial Components: IoT and AI-Enabled Innovation [Internet]. 2024 [cited 2025 May 8]. Available from: https://ieeexplore.ieee.org/abstract/document/10463472/
Elbadawi M, McCoubrey L, Gaisford S. Harnessing artificial intelligence for the next generation of 3D printed medicines [Internet]. 2021 [cited 2025 May 8]. Available from: https://www.sciencedirect.com/science/article/pii/S0169409X21001794
Zhou J, See CW, Sreenivasamurthy S, Zhu D. Customized additive manufacturing in bone scaffolds—the gateway to precise bone defect treatment [Internet]. 2023 [cited 2025 Jun 18]. Available from: https://spj.science.org/doi/abs/10.34133/research.0239
Rossi GD, Vergani L, Bertarelli F. A Novel Triad of Bio-Inspired Design, Digital Fabrication, and Bio-Derived Materials for Personalised Bone Repair [Internet]. 2024 [cited 2025 Jun 18]. Available from: https://www.mdpi.com/1996-1944/17/21/5305
Pathak K, Saikia R, Das A, Dutta D. 3D printing in biomedicine: Advancing personalized care through additive manufacturing [Internet]. 2023 [cited 2025 Jun 18]. Available from: https://www.explorationpub.com/Journals/em/Article/1001200
Harale AA, Jadhav DB, Jadhav PV, Mohite DD, Unde SS. Revolutionizing Orthopedics through Integration of Artificial Intelligence and 3D Printing for Enhanced Patient Care. Indian J Orthop. 2025. doi: 10.1007/s43465-025-01423-4.
Aziz NA, Adnan N, Wahab DA. Component design optimisation based on artificial intelligence in support of additive manufacturing repair and restoration: Current status and future outlook [Internet]. 2021 [cited 2025 May 8]. Available from: https://www.sciencedirect.com/science/article/pii/S0959652621006211
Omigbodun FT. A Review inside Innovation: AI and Additive Manufacturing for Advanced Bone Scaffold Design. IgMin Res. September 24, 2025; 3(9): 346-361. IgMin ID: igmin316; DOI:10.61927/igmin316; Available at: igmin.link/p316
Anyone you share the following link with will be able to read this content:
1Wolfson School of Mechanical, Electrical and Manufacturing Engineering, Loughborough University, UK
2The Manufacturing Technology Centre, Coventry, UK
Address Correspondence:
Francis T Omigbodun, Wolfson School of Mechanical, Electrical and Manufacturing Engineering, Loughborough University, UK, Email: [email protected]
How to cite this article:
Omigbodun FT. A Review inside Innovation: AI and Additive Manufacturing for Advanced Bone Scaffold Design. IgMin Res. September 24, 2025; 3(9): 346-361. IgMin ID: igmin316; DOI:10.61927/igmin316; Available at: igmin.link/p316
Copyright: © 2025 Omigbodun FT. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 Figure 1: Flowchart illustrating the major challenges in tra...
Figure 1: Flowchart illustrating the major challenges in tra...
![Conceptual map of current challenges in scaffold design and production, including material, biological, and technological barriers. Adapted from [19,25-29,61-68]](https://www.igminresearch.com/articles/figures/igmin316/igmin316.g002.png) Figure 2: Conceptual map of current challenges in scaffold d...
Figure 2: Conceptual map of current challenges in scaffold d...
 Figure 3: Comparative radar plot showing the benefits of AI ...
Figure 3: Comparative radar plot showing the benefits of AI ...
![Framework demonstrating how AI and AM can be integrated for clinical applications of bone scaffolds. Patient-specific imaging data flow into AI-driven design, which is then fabricated by AM and validated in vivo. Adapted from [80-85].](https://www.igminresearch.com/articles/figures/igmin316/igmin316.g004.png) Figure 4: Framework demonstrating how AI and AM can be integ...
Figure 4: Framework demonstrating how AI and AM can be integ...
![Parallel coordinates plot visualizing overlapping and unique challenges in AI, AM, and their combined use, including sustainability, equity, and scalability concerns [90-98].](https://www.igminresearch.com/articles/figures/igmin316/igmin316.g005.png) Figure 5: Parallel coordinates plot visualizing overlapping ...
Figure 5: Parallel coordinates plot visualizing overlapping ...
 Table 1: Comparative analysis of sustainability indicators ...
Table 1: Comparative analysis of sustainability indicators ...
 Table 2: Future trends in AI-AM scaffold design with innova...
Table 2: Future trends in AI-AM scaffold design with innova...
 Table 3: Strategic recommendations for advancing AI-AM in h...
Table 3: Strategic recommendations for advancing AI-AM in h...
Cong B, Zhang H. Innovative 3D printing technologies and advanced materials revolutionizing orthopedic surgery: current applications and future directions. Front Bioeng Biotechnol. 2025 Feb 11;13:1542179. doi: 10.3389/fbioe.2025.1542179. PMID: 40008034; PMCID: PMC11850356.
Pancholi S, Shaikh S, Kharbas S, Bhowmik S, Natarajan S, Khakhar D, et al. Transforming Additive Manufacturing with Artificial Intelligence: A Review of Current and Future Trends. Arch Comput Methods Eng. 2025. doi: 10.1007/S11831-025-10283-Y.
Ahalya K, Babu K-H. Revolutionizing biomedical engineering: Extrusion-based hydroxyapatite printing for scaffold construction: A review. Elsevier; 2024 [accessed 2025 Jun 18]. Available from: https://www.sciencedirect.com/science/article/pii/S2773207X24000885
Górski F. Computer Aided Design of 3D Printable Anatomically Shaped Medical Devices: Methodologies and Applications. 1st ed. CRC Press; 2025 Jan. doi: 10.1201/9781003386544/COMPUTER-AIDED-DESIGN-3D-PRINTABLE-ANATOMICALLY-SHAPED-MEDICAL-DEVICES-FILIP-GORSKI.
Helguero CG, Saldana SJ, Jimenez OC, DeFelipe M, Koutsou AD. Trabecular Scaffolds’ Mechanical Properties of Bone Reconstruction Using Biomimetic Implants. Procedia CIRP. 2017;65:121–6. doi: 10.1016/j.procir.2017.04.033.
Ambekar RS, Kandasubramanian B. Progress in the Advancement of Porous Biopolymer Scaffold: Tissue Engineering Application. Ind Eng Chem Res. 2019;58(16):6163–94. doi: 10.1021/acs.iecr.8b05334.
Zhou J, See CW, Sreenivasamurthy S, Zhu D. Customized Additive Manufacturing in Bone Scaffolds-The Gateway to Precise Bone Defect Treatment. Research (Wash D C). 2023 Oct 9;6:0239. doi: 10.34133/research.0239. PMID: 37818034; PMCID: PMC10561823.
Foroughi AH, Valeri C, Razavi MJ. A review of computational optimization of bone scaffold architecture: methods, challenges, and perspectives. Prog Biomed Eng. 2024 Nov 21;7(1). doi: 10.1088/2516-1091/ad879a. PMID: 39655853.
Zarei A, Faridmehr A. Synergizing additive manufacturing and machine learning for advanced hydroxyapatite scaffold design in bone regeneration. Springer; 2024 [accessed 2025 May 8]. Available from: https://link.springer.com/article/10.1007/s41779-024-01084-w
Pemmada R, Gowtham NH, Xia Y, Basu B, Thomas V. ML and AI approaches for design of tissue scaffolds. In: Thomas V, Basu B, editors. Artificial Intelligence in Tissue and Organ Regeneration. 1st ed. Elsevier; 2023. p. 29–56. doi: 10.1016/B978-0-443-18498-7.00008-9.
Katti DR, Katti KS, Gaikwad HK, Jaswandkar SV. Artificial intelligence in multiscale scaffolds for cancer organoids testbed. In: Thomas V, Basu B, editors. Artificial Intelligence in Tissue and Organ Regeneration. 1st ed. Elsevier; 2023. p. 193–218. doi: 10.1016/B978-0-443-18498-7.00005-3.
Heljak MK, Kijeńska-Gawrońska E, Swieszkowski W. Computational methods in modeling of scaffolds for neural tissue engineering. In: Pawar PV, editor. Biomaterials for Neural Tissue Engineering. 1st ed. Elsevier; 2023. p. 201–19. doi: 10.1016/B978-0-323-90554-1.00002-1.
Baino F, Ferraris S, Baino E, Vitale-Brovarone C. Recent trends in design, manufacturing and challenges of bone-like bioceramic scaffolds. Ceram Int. 2025 Apr. doi: 10.1016/J.CERAMINT.2025.02.307.
Ibrahimi S, D’Andrea L, Gastaldi D, Rivolta MW, Vena P. Machine learning approaches for the design of biomechanically compatible bone tissue engineering scaffolds. Comput Methods Appl Mech Eng. 2024 Apr;423:116842. doi: 10.1016/j.cma.2024.116842.
Ibrahimi S, D’Andrea L, Gastaldi D, Rivolta MW, Vena P. Machine learning approaches for the design of biomechanically compatible bone tissue engineering scaffolds [Internet]. Amsterdam: Elsevier; 2024 [cited 2025 May 8]. Available from: https://www.sciencedirect.com/science/article/pii/S0045782524000987
Yuan X, Zhu W, Yang Z, He N, Chen F, Han X, Zhou K. Recent advances in 3D printing of smart scaffolds for bone tissue engineering and regeneration. Adv Mater. 2024 Aug;36(34):e2403641. doi: 10.1002/adma.202403641. Epub 2024 Jun 28. PMID: 38861754.
Necolau M, Ionita M, Popa A. Poly(propylene fumarate) composite scaffolds for bone tissue engineering: Innovation in fabrication techniques and artificial intelligence integration [Internet]. Basel: MDPI; 2025 [cited 2025 May 8]. Available from: https://www.mdpi.com/2073-4360/17/9/1212
AI-enhanced bioactive 3D-printed scaffolds for tissue regeneration: Innovations in healing and functional additives [Internet]. J Comput Biomed Inform. [cited 2025 May 8]. Available from: https://jcbi.org/index.php/Main/article/view/863
Zhang ZZ, Jiang D, Ding JX, Wang SJ, Zhang L, Zhang JY, Qi YS, Chen XS, Yu JK. Role of scaffold mean pore size in meniscus regeneration. Acta Biomater. 2016 Oct 1;43:314-26. doi: 10.1016/j.actbio.2016.07.050. Epub 2016 Jul 29. PMID: 27481291.
Subia B, Kundu J, Choudhury S. Biomaterial scaffold fabrication techniques for potential tissue engineering applications. Tissue Eng. 2010 Jun; [Internet]. doi: 10.5772/8581.
Collins MN, Ren G, Young K, Pina S, Reis RL, Oliveira JM. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv Funct Mater. 2021 May;31(21):2010609. doi: 10.1002/adfm.202010609.
Mahanani ES, Puspita S, Dewi AH. The proper design of scaffold porosity for bone regeneration (literature review). AIP Conf Proc. 2024 May;3127(1):3294469. doi: 10.1063/5.0215991/3294469.
Korpela J, Kokkari A, Korhonen H, Malin M, Närhi T, Seppälä J. Biodegradable and bioactive porous scaffold structures prepared using fused deposition modeling. J Biomed Mater Res B Appl Biomater. 2013 May;101(4):610-9. doi: 10.1002/jbm.b.32863. Epub 2012 Dec 20. PMID: 23281260.
Janik and M. Marzec, ‘A review: Fabrication of porous polyurethane scaffolds’, Materials Science and Engineering C, vol. 48, pp. 586–591, 2015, doi: 10.1016/j.msec.2014.12.037. Janik H, Marzec M. A review: fabrication of porous polyurethane scaffolds. Mater Sci Eng C Mater Biol Appl. 2015 Mar;48:586-91. doi: 10.1016/j.msec.2014.12.037. Epub 2014 Dec 17. PMID: 25579961.
Chong ETJ, Ng JW, Lee PC. Classification and medical applications of biomaterials–a mini review. BIO Integration. 2023 Aug;4(2):54. doi: 10.15212/BIOI-2022-0009.
Oladapo BI, Zahedi SA, Chong S, Omigbodun FT, Malachi IO. 3D printing of surface characterisation and finite element analysis improvement of PEEK-HAP-GO in bone implant. 2019 [cited 2024 Oct 24]. Available from: https://dora.dmu.ac.uk/bitstream/2086/18915/3/3D%20printing%20of%20surface%20characterisation%20and%20finite%20element%20analysis%20improvement%20of%20tensile%20properties%20for%20PEEK-HAP-GO%20in%20bone%20implant.pdf
Oladapo BI, Zahedi SA, Chong S, Omigbodun FT, Malachi IO. 3D printing of PEEK–cHAp scaffold for medical bone implant. Biodes Manuf. 2021 Mar;4(1):44–59. doi: 10.1007/S42242-020-00098-0.
Zhao Y, Zhao K, Li Y, Chen F. Mechanical characterization of biocompatible PEEK by FDM. J Manuf Process. 2020 Apr;56:28–42. doi: 10.1016/j.jmapro.2020.04.063.
Olawumi MA, Omigbodun FT, Oladapo BI, Olugbade TO, Olawade DB. Innovative PEEK in dentistry of enhanced adhesion and sustainability through AI-driven surface treatments. Bioengineering (Basel). 2024 Sep 14;11(9):924. doi: 10.3390/bioengineering11090924. PMID: 39329666; PMCID: PMC11429295.
Oladapo BI, Ismail SO, Bowoto OK, Omigbodun FT, Olawumi MA, Muhammad MA. Lattice design and 3D-printing of PEEK with Ca10(OH)(PO4)3 and in-vitro bio-composite for bone implant. Int J Biol Macromol. 2020 Dec 15;165(Pt A):50–62. doi: 10.1016/j.ijbiomac.2020.09.175. PMID: 32979443.
Zhang L, Morsi Y, Wang Y, Li Y, Ramakrishna S. Review scaffold design and stem cells for tooth regeneration. Jpn Dent Sci Rev. 2013;49(1):14–26. doi: 10.1016/j.jdsr.2012.09.001.
Haghdan S, Smith GD. Natural fiber reinforced polyester composites: a literature review. 2015 Jul 11. doi: 10.1177/0731684415588938.
Siakeng R, Jawaid M, Ariffin H, Sapuan SM, Asim M, Saba N. Natural fiber reinforced polylactic acid composites: a review. Polym Compos. 2019;40(2):446–63. doi: 10.1002/pc.24747.
Omigbodun F. Manufacturing and mechanical characterization of PLA/cHAP/rGO composite for bone implants. 2024 Nov. doi: 10.26174/THESIS.LBORO.27867627.V1.
Omigbodun FT, Oladapo BI. Enhanced mechanical properties and degradation control of poly(lactic) acid/hydroxyapatite/reduced graphene oxide composites for advanced bone tissue engineering application. Biomimetics (Basel). 2024 Oct 23;9(11):651. doi: 10.3390/biomimetics9110651. PMID: 39590223; PMCID: PMC11592037.
Omigbodun FT, Oladapo BI, Osa-uwagboe N. Exploring the frontier of polylactic acid/hydroxyapatite composites in bone regeneration and their revolutionary biomedical applications – a review. J Reinf Plast Compos. 2024 Sep. doi: 10.1177/07316844241278045. Available from: https://journals.sagepub.com/doi/pdf/10.1177/07316844241278045
Omigbodun FT, Bowoto OK, Nimako PA, Elemure IE, Ojo GG. Reduction in economic cost and production time for development of a 3D printer and its effect on market economic. Int J Eng Trends Technol. 2019;67(6). doi: 10.14445/22315381/IJETT-V67I6P218.
Omigbodun F, Osa-Uwagboe N, Udu A, Oladapo BO. Leveraging machine learning for optimized mechanical properties and 3D printing of PLA/cHAP for bone implant. Biomimetics (Basel). 2024 [cited 2024 Oct 24]. Available from: https://www.mdpi.com/2313-7673/9/10/587
Oladapo BI, Zahedi SA, Omigbodun FT. A systematic review of polymer composite in biomedical engineering. Eur Polym J. 2021;154:110534. doi: 10.1016/j.eurpolymj.2021.110534.
Appuhamillage G, Ahmad SA, et al. 3D and 4D printing of biomedical materials: current trends, challenges, and future outlook. 2024 [cited 2025 Jun 18]. Available from: https://www.explorationpub.com/Journals/em/Article/1001203
Zhou J, See CW, Sreenivasamurthy S, Zhu D. Customized Additive Manufacturing in Bone Scaffolds-The Gateway to Precise Bone Defect Treatment. Research (Wash D C). 2023 Oct 9;6:0239. doi: 10.34133/research.0239. PMID: 37818034; PMCID: PMC10561823.
Kumar P, Rajak D, Abubakar M, Singh SA. 3D printing technology for biomedical practice: a review. J Mater Eng Perform. 2021. Available from: https://link.springer.com/article/10.1007/s11665-021-05792-3
Oladapo BI, Ismail SO, Omigbodun FT, Oluwole B, Olawumi MA, Samad YA. 4D Printing of Nanostructure Modified Shape Memory Polymer Composites. 2024. Available from: https://researchprofiles.herts.ac.uk/en/publications/4d-printing-of-nanostructure-modified-shape-memory-polymer-compos
Mehrpouya M, Vahabi H, Janbaz S, Darafsheh A, Mazur TR, Ramakrishna S. 4D printing of shape memory polylactic acid (PLA). Polymer (Guildf). 2021 Aug;230:124080. doi: 10.1016/j.polymer.2021.124080.
Ngo TD, Kashani A, Imbalzano G, Nguyen KTQ, Hui D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos B Eng. 2018 Feb;143:172–196. doi: 10.1016/j.compositesb.2018.02.012.
Wang DD, Qian Z, Vukicevic M, Engelhardt S, Kheradvar A, Zhang C, et al. 3D Printing, Computational Modeling, and Artificial Intelligence for Structural Heart Disease. JACC Cardiovasc Imaging. 2021 Jan;14(1):41-60. doi: 10.1016/j.jcmg.2019.12.022. Epub 2020 Aug 26. PMID: 32861647.
Muhindo D, Elkanayati R, Srinivasan P, Repka MA, Ashour EA. Recent Advances in the Applications of Additive Manufacturing (3D Printing) in Drug Delivery: A Comprehensive Review. AAPS PharmSciTech. 2023 Feb 9;24(2):57. doi: 10.1208/s12249-023-02524-9. Erratum in: AAPS PharmSciTech. 2023 Mar 10;24(3):75. doi: 10.1208/s12249-023-02542-7. PMID: 36759435.
Choi WJ, Hwang KS, Kwon HJ, Lee C, Kim CH, Kim TH, et al. Rapid development of dual porous poly(lactic acid) foam using fused deposition modeling (FDM) 3D printing for medical scaffold application. Mater Sci Eng C Mater Biol Appl. 2020 May;110:110693. doi: 10.1016/j.msec.2020.110693. Epub 2020 Jan 27. PMID: 32204007.
Ruiz-Alonso S, Lafuente-Merchan M, Ciriza J, Saenz-Del-Burgo L, Pedraz JL. Tendon tissue engineering: Cells, growth factors, scaffolds and production techniques. J Control Release. 2021 May 10;333:448-486. doi: 10.1016/j.jconrel.2021.03.040. Epub 2021 Mar 31. PMID: 33811983.
Turnbull G, Clarke J, Picard F, Riches P, Jia L, Han F, et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018 Sep;3(3):278–314. doi: 10.1016/j.bioactmat.2017.10.001.
Wang C, Wang M, Xu T, Zhang X, Lin K. 3D printing of bone tissue engineering scaffolds. Bioact Mater. 2020 Mar;5(1):82–91. doi: 10.1016/j.bioactmat.2020.01.004.
Farah S, Anderson DG, Langer R. Physical and mechanical properties of PLA, and their functions in widespread applications - A comprehensive review. Adv Drug Deliv Rev. 2016 Dec 15;107:367-392. doi: 10.1016/j.addr.2016.06.012. Epub 2016 Jun 26. PMID: 27356150.
Penumakala PK, Santo J, Thomas A. A critical review on the fused deposition modeling of thermoplastic polymer composites. Compos B Eng. 2020 Jul;201:108336. doi: 10.1016/j.compositesb.2020.108336.
Wang Y, Zhou Y, Lin L, Corker J, Fan M. Overview of 3D additive manufacturing (AM) and corresponding AM composites. Compos Part A Appl Sci Manuf. 2020 Jul;139:106114. doi: 10.1016/j.compositesa.2020.106114.
Almesmari A, Sheikh-Ahmad J, Jarrar F, Bojanampati S. Optimizing the specific mechanical properties of lattice structures fabricated by material extrusion additive manufacturing. J Mater Res Technol. 2023 Jan;22:1821–1838. doi: 10.1016/j.jmrt.2022.12.024.
Fedorenko A, Fedulov B, Jurgenson S, Lomakin E. 3D lattice-based structural elements for industrial application. Procedia Struct Integr. 2021;31:652–657. doi: 10.1016/j.prostr.2021.10.072.
Chawla T, Rukhaiyar V, Banu SA, Singh S. The future of tissue engineering: Integrating ML, AI, and computer vision. 2025. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003536352-2/future-tissue-engineering-tvisha-chawla-vaishnavi-rukhaiyar-shaik-arshid-banu-subhash-singh
Basu B, Gowtham N, Xiao Y, Kalidindi S, Lim K. Biomaterialomics: Data science-driven pathways to develop fourth-generation biomaterials. Acta Biomater. 2022. Available from: https://www.sciencedirect.com/science/article/pii/S1742706122001039
Rossi GD, Vergani L, Bonfanti F. A Novel Triad of Bio-Inspired Design, Digital Fabrication, and Bio-Derived Materials for Personalised Bone Repair. Materials (Basel). 2024. Available from: https://www.mdpi.com/1996-1944/17/21/5305
Pancholi S, Gupta M, Bansal M. Transforming Additive Manufacturing with Artificial Intelligence: A Review of Current and Future Trends. 2025. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003536352-2/future-tissue-engineering-tvisha-chawla-vaishnavi-rukhaiyar-shaik-arshid-banu-subhash-singh
Basu B, Gowtham N, Xiao Y, Kalidindi S. Biomaterialomics: Data science-driven pathways to develop fourth-generation biomaterials. Acta Biomater. 2022. Available from: https://www.sciencedirect.com/science/article/pii/S1742706122001039
Cong B, Zhao H. Innovative 3D printing technologies and advanced materials revolutionizing orthopedic surgery: current applications and future directions. Front Bioeng Biotechnol. 2025. Available from: https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1542179/full
Gorski F. Computer Aided Design of 3D Printable Anatomically Shaped Medical Devices: Methodologies and Applications. 2025. Available from: https://www.taylorfrancis.com/books/mono/10.1201/9781003386544/computer-aided-design-3d-printable-anatomically-shaped-medical-devices-filip-gorski
Banerjee A, Haridas H, Sen A. Artificial intelligence in 3D printing: a revolution in health care. In: Applications of 3D Printing in Healthcare. Springer; 2021. Available from: https://link.springer.com/chapter/10.1007/978-981-33-6703-6_4
Kumar N, Chakradhar D. Advancing bioimplant manufacturing through artificial intelligence. In: Bioimplants Manufacturing: Fundamentals and Advances. CRC Press; 2024. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003509943-13/advancing-bioimplant-manufacturing-artificial-intelligence-namadi-vinod-kumar-chakradhar-abhilash
Verma D, Dong Y, Sharma M, Saini M. Advanced processing of 3D printed biocomposite materials using artificial intelligence. Mater Manuf Process. 2022;37(5):518–538. Available from: https://www.tandfonline.com/doi/abs/10.1080/10426914.2021.1945090
Mittal R, Haleem A, Kumar A. Advances in additive manufacturing: artificial intelligence, nature-inspired, and biomanufacturing. 2022. Available from: https://books.google.com/books?hl=en&lr=&id=c8h6EAAAQBAJ&oi=fnd&pg=PP1
Monfred V. Application of Artificial Intelligence (Machine Learning) in Additive Manufacturing, Bio-Systems, Bio-Medicine, and Composites. In: Additive Manufacturing for Biocomposites and Synthetic Composites. CRC Press; 2023. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003362128-9
Khan SB, Irfan S, Zhang Z, Yuan W. Redefining Medical Applications with Safe and Sustainable 3D Printing. ACS Appl Bio Mater. 2025 Aug 18;8(8):6470–6525. doi: 10.1021/acsabm.4c01923. Epub 2025 Apr 8. PMID: 40200689. Available from: https://pubs.acs.org/doi/10.1021/acsabm.4c01923
Banerjee A, Haridas HK, SenGupta A, Jabalia N. Artificial Intelligence in 3D Printing: A Revolution in Health Care. In: Lecture Notes in Bioengineering. 2022:57–79. doi: 10.1007/978-981-33-6703-6_4. Available from: https://link.springer.com/chapter/10.1007/978-981-33-6703-6_4
Kumar NV, Chakradhar D, Abhilash PM. Advancing bioimplant manufacturing through artificial intelligence. In: Bioimplants Manufacturing: Fundamentals and Advances. 2024:284–312. doi: 10.1201/9781003509943-13. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003509943-13
Rojek I, Mikołajewski D, Dostatni E, Mazur M. AI-optimized technological aspects of the material used in 3D printing processes for selected medical applications. Materials (Basel). 2020;13(23):5437. Available from: https://www.mdpi.com/1996-1944/13/23/5437
Banerjee A, Haridas H, Sen A. Artificial intelligence in 3D printing: a revolution in health care. In: Applications of 3D Printing in Healthcare. Springer; 2021. Available from: https://link.springer.com/chapter/10.1007/978-981-33-6703-6_4
Wang Y, Zheng P, Peng T, Yang H, Zou J. Smart additive manufacturing: Current artificial intelligence-enabled methods and future perspectives. Sci China Technol Sci. 2020;63(9):1600–1611. Available from: https://link.springer.com/article/10.1007/s11431-020-1581-2
Yang J, Chen Y, Huang W, Liu Y. Survey on artificial intelligence for additive manufacturing. In: 2027 23rd International Conference on Document Analysis and Recognition (ICDAR). 2017. Available from: https://ieeexplore.ieee.org/abstract/document/8082053/
Goh G, Sing S, Yeong WY. A review on machine learning in 3D printing: applications, potential, and challenges. Artif Intell Rev. 2020;54(1):1–32. doi: 10.1007/s10462-020-09876-9. Available from: https://link.springer.com/article/10.1007/s10462-020-09876-9
Rodríguez-Espíndola O, Chowdhury S, Beltagui A, Albores P. The potential of emergent disruptive technologies for humanitarian supply chains: the integration of blockchain, Artificial Intelligence and 3D printing. Int J Prod Res. 2020;58(15):4610–4630. doi: 10.1080/00207543.2020.1761565. Available from: https://doi.org/10.1080/00207543.2020.1761565
Jin Z, Zhang Z, Guo G. Automated real‐time detection and prediction of interlayer imperfections in additive manufacturing processes using artificial intelligence. AI Systems. 2019;2(1). doi: 10.1002/aisy.201900130. Available from: https://onlinelibrary.wiley.com/doi/10.1002/aisy.201900130
Wang YB, Zheng P, Peng T, Yang HY, Zou J. Smart additive manufacturing: Current artificial intelligence-enabled methods and future perspectives. Sci China Technol Sci. 2020;63(9):1600–1611. doi: 10.1007/S11431-020-1581-2. Available from: https://link.springer.com/article/10.1007/s11431-020-1581-2
Yuan X, Zhu W, Yang Z, He N, Cheng F. Recent advances in 3D printing of smart scaffolds for bone tissue engineering and regeneration. Adv Mater. 2024. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.202403641
Zarei A, Farazin A. Synergizing additive manufacturing and machine learning for advanced hydroxyapatite scaffold design in bone regeneration. J Aust Ceram Soc. 2024. doi: 10.1007/S41779-024-01084-W. Available from: https://link.springer.com/article/10.1007/s41779-024-01084-w
Omigbodun FT, Oladapo BI. AI-Optimized Lattice Structures for Biomechanics Scaffold Design. Biomimetics (Basel). 2025 Feb 1;10(2):88. doi: 10.3390/biomimetics10020088. PMID: 39997111; PMCID: PMC11853473. Available from: https://www.mdpi.com/1996-1944/10/2/88
Verma D, Dong Y, Sharma M, Chaudhary AK. Advanced processing of 3D printed biocomposite materials using artificial intelligence. Mater Manuf Process. 2022;37(5):518–538. doi: 10.1080/10426914.2021.1945090. Available from: https://www.tandfonline.com/doi/abs/10.1080/10426914.2021.1945090
Monfred V. Application of artificial intelligence (machine learning) in additive manufacturing, bio-systems, bio-medicine, and composites. In: Additive Manufacturing for Biocomposites and Synthetic Composites. 2023:152–203. doi: 10.1201/9781003362128-9. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003362128-9
Mahmood M, Visan A, Ristoscu C, Mihailescu IN. Artificial neural network algorithms for 3D printing. Materials (Basel). 2020;14(1):163. Available from: https://www.mdpi.com/1996-1944/14/1/163
Zhu Z, Ng D, Park H, Materials MM-NR, et al. 3D-printed multifunctional materials enabled by artificial-intelligence-assisted fabrication technologies. Nat Rev Mater. 2021 [cited 2025 May 8]. Available from: https://www.nature.com/articles/s41578-020-00235-2
Xin H, Virk AS, Virk SS, Akin-Ige F, Amin S. Applications of artificial intelligence and machine learning on critical materials used in cosmetics and personal care formulation design. Curr Opin Colloid Interface Sci. 2024 Oct;73:101847. doi: 10.1016/j.cocis.2024.101847
Cong B, Zhang H. Innovative 3D printing technologies and advanced materials revolutionizing orthopedic surgery: current applications and future directions. Front Bioeng Biotechnol. 2025 Feb 11;13:1542179. doi: 10.3389/fbioe.2025.1542179. PMID: 40008034; PMCID: PMC11850356
Li Z, Song P, Li G, Han Y, Ren X, Bai L, Su J. AI energized hydrogel design, optimization and application in biomedicine. Mater Today Bio. 2024 Feb 29;25:101014. doi: 10.1016/j.mtbio.2024.101014. PMID: 38464497; PMCID: PMC10924066
Murali A, Parameswaran R. Extrusion 3D printing advances for craniomaxillofacial bone tissue engineering. Polym Plast Technol Mater. 2024;63(7):889–912. doi: 10.1080/25740881.2024.2307351
Mohammadnabi S, Moslemy N, Taghvaei H, Zia AW, Askarinejad S, Shalchy F. Role of artificial intelligence in data-centric additive manufacturing processes for biomedical applications. J Mech Behav Biomed Mater. 2025 Jun;166:106949. doi: 10.1016/j.jmbbm.2025.106949. Epub 2025 Feb 25. PMID: 40036906
Cong B, Zhang H. Innovative 3D printing technologies and advanced materials revolutionizing orthopedic surgery: current applications and future directions. Front Bioeng Biotechnol. 2025 Feb 11;13:1542179. doi: 10.3389/fbioe.2025.1542179. PMID: 40008034; PMCID: PMC11850356
Khan S, Irfan S, Zhang Z, Materials WYAAB, et al. Redefining medical applications with safe and sustainable 3D printing. ACS Appl Bio Mater. 2025 [cited 2025 Jun 18]. Available from: https://pubs.acs.org/doi/abs/10.1021/acsabm.4c01923
Tripathi S, Ansari A, Singh M, Dash M, P K-M, et al. Surgical planning and procedures through synergistic use of additive manufacturing, advanced materials and artificial intelligence: challenges and opportunities. Mater Horiz. 2025 [cited 2025 Jun 18]. Available from: https://pubs.rsc.org/en/content/articlelanding/2025/mh/d5mh00501a
Jeyaraman M, Nallakumarasamy A, Jeyaraman N. Industry 5.0 in orthopaedics. Indian J Orthop. 2022 Aug 23;56(10):1694–1702. doi: 10.1007/s43465-022-00712-6. PMID: 36187596; PMCID: PMC9485301
Ikhsan R, Rahayu S, R A-AJRI, et al. Integrating artificial intelligence with 3D printing technology in healthcare: sustainable solutions for clinical training optimization. Asian J Res Innov. 2025;6(2):99–107. doi: 10.34306/ajri.v6i2.1126
Zhou J, See C, Sreenivasamurthy S, Zhu D. Customized additive manufacturing in bone scaffolds—the gateway to precise bone defect treatment. Research. 2023 [cited 2025 Jun 18]. Available from: https://spj.science.org/doi/abs/10.34133/research.0239
Sharma G, Rawat K, S S-IE, et al. Adoption of controls and saga of development in 3D printed bioimplants: a business perspective. IEEE Eng. 2024 [cited 2025 Jun 18]. Available from: https://ieeexplore.ieee.org/abstract/document/10683896/
Zhou J, See CW, Sreenivasamurthy S, Zhu D. Customized additive manufacturing in bone scaffolds—The gateway to precise bone defect treatment. Research (Wash D C). 2023 Oct 9;6:0239. doi: 10.34133/research.0239. PMID: 37818034; PMCID: PMC10561823
Chawla T, Rukhaiyar V, Banu SA, Singh S, et al. The future of tissue engineering: integrating ML, AI, and computer vision. In: Advances in Tissue Engineering. Taylor & Francis; 2025 [cited 2025 Jun 18]. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003536352-2/future-tissue-engineering-tvisha-chawla-vaishnavi-rukhaiyar-shaik-arshid-banu-subhash-singh
Harale A, Jadhav D, Jadhav P, et al. Revolutionizing orthopedics through integration of artificial intelligence and 3D printing for enhanced patient care. Springer. 2025 [cited 2025 Jun 18]. Available from: https://link.springer.com/article/10.1007/s43465-025-01423-4
Muhindo D, Elkanayati R, Srinivasan P, et al. Recent advances in the applications of additive manufacturing (3D printing) in drug delivery: a comprehensive review. Springer. 2023 [cited 2025 May 8]. Available from: https://link.springer.com/article/10.1208/s12249-023-02524-9
J. Exploring the interrelationship between additive manufacturing and Industry 4.0. Designs. 2020 [cited 2025 May 8]. Available from: https://www.mdpi.com/2411-9660/4/2/13
Xiong Y, Tang Y, Zhou Q, Ma Y, Ren D. Intelligent additive manufacturing and design: state of the art and future perspectives. Addit Manuf. 2022 [cited 2025 May 8]. Available from: https://www.sciencedirect.com/science/article/pii/S2214860422005280
Chien C-F, Dauzère-Pérès S, Huh WT, Jang YJ, Morrison JR. Artificial intelligence in manufacturing and logistics systems: algorithms, applications, and case studies. Int J Prod Res. 2020 May;58(9):2730–1. doi: 10.1080/00207543.2020.1752488
Rodríguez-Espíndola O, Chowdhury S, Beltagui A, Albores P. The potential of emergent disruptive technologies for humanitarian supply chains: the integration of blockchain, artificial intelligence and 3D printing. Int J Prod Res. 2020 [cited 2025 May 8]. Available from: https://www.tandfonline.com/doi/abs/10.1080/00207543.2020.1761565
Murali A, Prabhu R. Extrusion 3D printing advances for craniomaxillofacial bone tissue engineering. Prog Addit Manuf. 2024 [cited 2025 May 8]. Available from: https://www.tandfonline.com/doi/abs/10.1080/25740881.2024.2307351
Zhou J, See C, Sreenivasamurthy S, Zhu D. Customized additive manufacturing in bone scaffolds—the gateway to precise bone defect treatment. Research. 2023 [cited 2025 May 8]. Available from: https://spj.science.org/doi/abs/10.34133/research.0239
Kolomenskaya E, Butova V, Poltavskiy A, Smirnov A. Application of artificial intelligence at all stages of bone tissue engineering. Biomedicines. 2023 [cited 2025 May 8]. Available from: https://www.mdpi.com/2227-9059/12/1/76
Eber P, Sillmann Y, Guastaldi FG. Beyond 3D printing: how AI is shaping the future of craniomaxillofacial bone tissue engineering. ACS Biomater Sci Eng. 2025 [cited 2025 May 8]. Available from: https://pubs.acs.org/doi/abs/10.1021/acsbiomaterials.5c00420
Eber P, Sillmann YM, Guastaldi FPS. Beyond 3D printing: how AI is shaping the future of craniomaxillofacial bone tissue engineering. ACS Biomater Sci Eng. 2025 Jun 9;11(6):3095–8. doi: 10.1021/acsbiomaterials.5c00420. Epub 2025 May 2. PMID: 40314572.
Ramachandran MK, Raigar J, Kannan M, Velu R. State-of-the-art overview and recent trends in biomedical devices using digital manufacturing: opportunities, limitations, and current market. In: Digital Design and Manufacturing of Medical Devices and Systems. 2023:1–31. doi: 10.1007/978-981-99-7100-8_1.
Kennedy SM, K A, JJB JJ, V E, RB JR. Transformative applications of additive manufacturing in biomedical engineering: bioprinting to surgical innovations. J Med Eng Technol. 2024 May;48(4):151–68. doi: 10.1080/03091902.2024.2399017. Epub 2024 Sep 16. PMID: 39282861.
Shokrani H, et al. Artificial intelligence for biomedical engineering of polysaccharides: a short overview. Curr Opin Biomed Eng. 2023 Sep;27:100463. doi: 10.1016/J.COBME.2023.100463.
Thomas V, Chandy T, Sharma CP. AI and ML: challenges and future perspective in artificial organs realm. In: Artificial Intelligence in Tissue and Organ Regeneration. 2023 Jan:303–16. doi: 10.1016/B978-0-443-18498-7.00015-6.
Wu C, et al. Topology optimisation for design and additive manufacturing of functionally graded lattice structures using derivative-aware machine learning algorithms. Addit Manuf. 2023 Sep;78:103833. doi: 10.1016/J.ADDMA.2023.103833.
Lin F, Basem A, Khaddour MH, Salahshour S, Li W, Sabetvand R. Advancing mechanical and biological characteristics of polymer-ceramic nanocomposite scaffolds for sport injuries and bone tissue engineering: a comprehensive investigation applying finite element analysis and artificial neural network. Ceram Int. 2025 Apr. doi: 10.1016/J.CERAMINT.2025.01.115.
Ramesh S, Deep A, Tamayol A, Kamaraj A, Mahajan C, Madihally S. Advancing 3D bioprinting through machine learning and artificial intelligence. Bioprinting. 2024 Apr;38:e00331. doi: 10.1016/J.BPRINT.2024.E00331.
Wu C, Wan B, Entezari A, Fang J, Xu Y, Li Q. Machine learning-based design for additive manufacturing in biomedical engineering. Int J Mech Sci. 2024 Mar;266:108828. doi: 10.1016/J.IJMECSCI.2023.108828.
Li Z, et al. Inverse design of cellular structures with the geometry of triply periodic minimal surfaces using generative artificial intelligence algorithms. Eng Struct. 2024 Dec;321:118988. doi: 10.1016/J.ENGSTRUCT.2024.118988.
Khan N, Asad H, Khan S, Riccio A. Towards defect-free lattice structures in additive manufacturing: a holistic review of machine learning advancements. J Manuf Process. 2025 Jun;144:1–53. doi: 10.1016/J.JMAPRO.2025.04.035.
Greenberg ZF, Graim KS, He M. Towards artificial intelligence-enabled extracellular vesicle precision drug delivery. Adv Drug Deliv Rev. 2023 Aug;199:114974. doi: 10.1016/j.addr.2023.114974. Epub 2023 Jun 23. PMID: 37356623.
Li W, Li L, Hu J, Zhou D, Su H. Design and Applications of Supramolecular Peptide Hydrogel as Artificial Extracellular Matrix. Biomacromolecules. 2024 Nov 11;25(11):6967-6986. doi: 10.1021/acs.biomac.4c00971. Epub 2024 Oct 17. PMID: 39418328.
Kanthimathi T, Rathika N, Fathima A. Robotic 3D Printing for Customized Industrial Components: IoT and AI-Enabled Innovation [Internet]. 2024 [cited 2025 May 8]. Available from: https://ieeexplore.ieee.org/abstract/document/10463472/
Elbadawi M, McCoubrey L, Gaisford S. Harnessing artificial intelligence for the next generation of 3D printed medicines [Internet]. 2021 [cited 2025 May 8]. Available from: https://www.sciencedirect.com/science/article/pii/S0169409X21001794
Zhou J, See CW, Sreenivasamurthy S, Zhu D. Customized additive manufacturing in bone scaffolds—the gateway to precise bone defect treatment [Internet]. 2023 [cited 2025 Jun 18]. Available from: https://spj.science.org/doi/abs/10.34133/research.0239
Rossi GD, Vergani L, Bertarelli F. A Novel Triad of Bio-Inspired Design, Digital Fabrication, and Bio-Derived Materials for Personalised Bone Repair [Internet]. 2024 [cited 2025 Jun 18]. Available from: https://www.mdpi.com/1996-1944/17/21/5305
Pathak K, Saikia R, Das A, Dutta D. 3D printing in biomedicine: Advancing personalized care through additive manufacturing [Internet]. 2023 [cited 2025 Jun 18]. Available from: https://www.explorationpub.com/Journals/em/Article/1001200
Harale AA, Jadhav DB, Jadhav PV, Mohite DD, Unde SS. Revolutionizing Orthopedics through Integration of Artificial Intelligence and 3D Printing for Enhanced Patient Care. Indian J Orthop. 2025. doi: 10.1007/s43465-025-01423-4.
Aziz NA, Adnan N, Wahab DA. Component design optimisation based on artificial intelligence in support of additive manufacturing repair and restoration: Current status and future outlook [Internet]. 2021 [cited 2025 May 8]. Available from: https://www.sciencedirect.com/science/article/pii/S0959652621006211