
Unveiling the Hidden Beat: Heart Rate Variability and the Vagus Nerve as an Emerging Biomarker in Breast Cancer Management
CardiologyReceived 16 Jul 2025 Accepted 09 Aug 2025 Published online 11 Aug 2025
ISSN: 2995-8067 | Quick Google Scholar
Previous Full Text
Human Missions to Mars Using the Starship


Received 16 Jul 2025 Accepted 09 Aug 2025 Published online 11 Aug 2025
Heart rate variability (HRV), a non-invasive measure of autonomic nervous system (ANS) function reflecting vagus nerve activity, is a promising biomarker in breast cancer (BC) management. This PRISMA-guided systematic review evaluates HRV electrophysiologic data from 5-minute vs. 24-hour ECG recordings in BC diagnosis and therapy follow-up, emphasizing the vagus nerve’s role in inflammation and tumor progression. Completing a search of PubMed, Scopus, Web of Science, and Embase (2009 - 2025), 16 studies (n = 3,412 participants) were included. Lower HRV metrics (e.g., SDNN < 50 ms, RMSSD < 20 ms predicted relapse) correlated with advanced BC stages, elevated carcinoembryonic antigen (CEA), and poorer prognosis (HR = 0.62, 95% CI: 0.48 - 0.79). Chemotherapy-induced HRV reductions (e.g., SDNN decrease by 20%) predicted cardiotoxicity, while vagus nerve stimulation (VNS) improved HRV and reduced inflammation. HRV showed diagnostic sensitivity up to 80% with biomarkers. Additionally, meta-analysis was precluded due to significant methodological heterogeneity across studies. A narrative synthesis was conducted due to this heterogeneity; meta-analysis was not feasible due to inconsistent HRV protocols and outcome measures. HRV and vagal interventions hold transformative potential, necessitating standardized protocols and larger studies.
Breast cancer (BC), with an estimated 2.3 million new cases annually, stands as the most prevalent malignancy among women globally, accounting for approximately 25% of all cancer diagnoses []. The disease encompasses a spectrum of histological subtypes, including ductal carcinoma, lobular carcinoma, and rarer forms, with staging ranging from in situ (stage 0) to metastatic (stage IV) based on tumor size, lymph node involvement, and distant metastasis []. Diagnosis typically begins with clinical breast examination and imaging modalities such as mammography, ultrasound, and magnetic resonance imaging (MRI), which detect morphological abnormalities []. Definitive diagnosis relies on biopsy, with histopathological analysis confirming malignancy and receptor status (e.g., estrogen receptor [ER], progesterone receptor [PR], human epidermal growth factor receptor 2 [HER2]) to guide personalized treatment []. Follow-up care is equally critical, involving regular imaging, tumor marker assessments (e.g., carcinoembryonic antigen [CEA], cancer antigen 15-3 []), and monitoring for recurrence or therapy-related toxicities, such as cardiotoxicity from anthracyclines or trastuzumab []. Despite advances in early detection and targeted therapies, challenges persist, including late-stage diagnoses in resource-limited settings and the long-term impact of treatment on patient quality of life, necessitating innovative biomarkers for improved management.
Heart rate variability (HRV) represents an electrophysiologic measurement derived from the electrocardiogram (ECG), which records the electrical activity of the heart over time []. HRV quantifies the beat-to-beat variations in R-R intervals (the time between consecutive R waves), reflecting the dynamic interplay between the sympathetic and parasympathetic branches of the autonomic nervous system (ANS) []. Standard HRV parameters include time-domain measures such as the standard deviation of NN intervals (SDNN) and the root mean square of successive differences (RMSSD), which indicate overall variability and parasympathetic activity, respectively, as well as frequency-domain measures like high-frequency (HF) power (0.15 - 0.4 Hz), a marker of vagal tone, and low-frequency (LF) power (0.04 - 0.15 Hz), influenced by both sympathetic and parasympathetic inputs []. These metrics are typically extracted from short-term (e.g., 5-minute) or long-term (e.g., 24-hour) ECG recordings, with the choice of duration affecting the sensitivity and specificity of the findings []. HRV’s non-invasive nature, combined with its ability to capture subtle autonomic changes, positions it as a promising tool for assessing physiological states beyond cardiovascular health, particularly in the context of cancer (Figure 1).
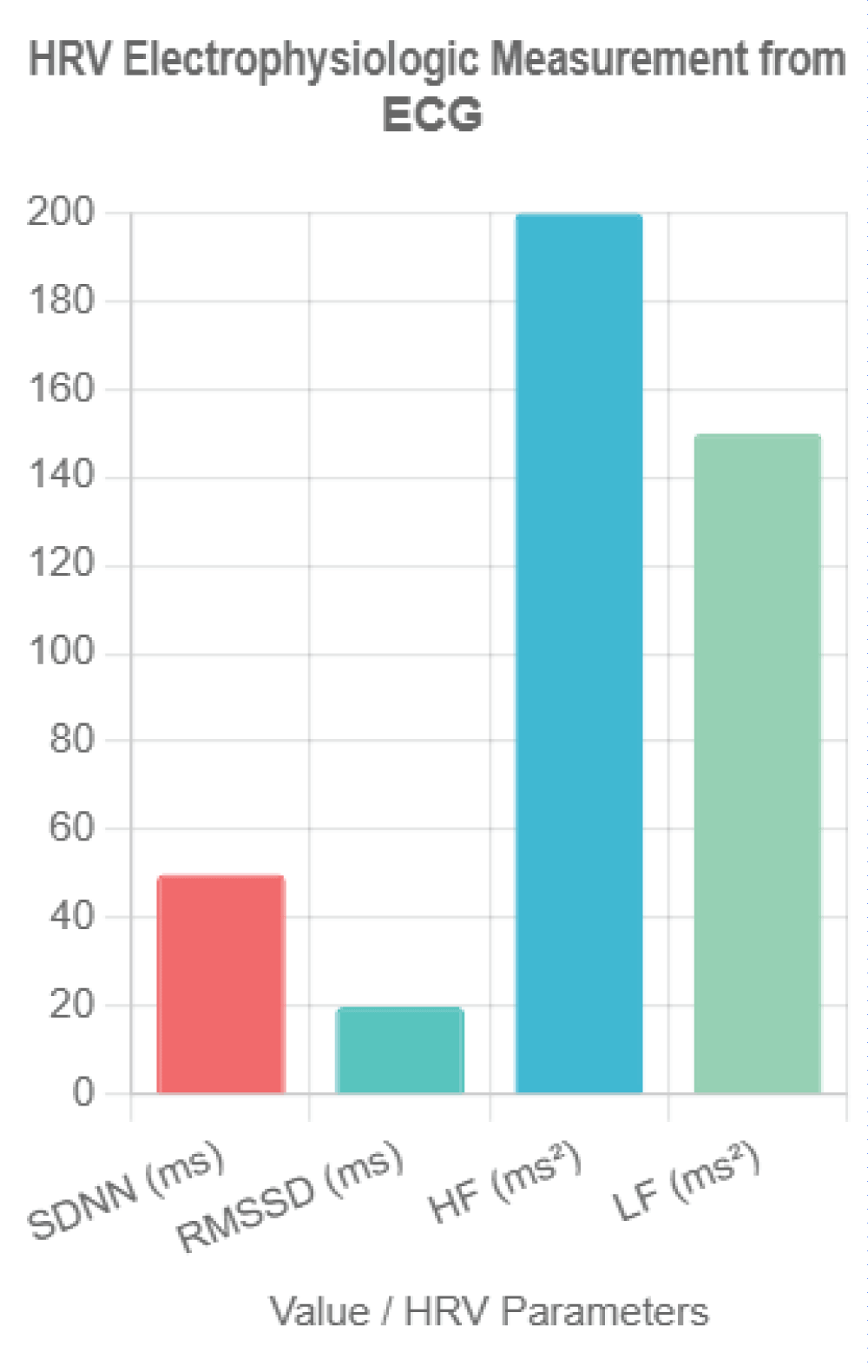 Figure 1: HRV Electrophysiologic Measurement from ECG. This bar chart illustrates key heart rate variability (HRV) parameters derived from electrocardiogram (ECG) recordings, including SDNN (standard deviation of NN intervals) in milliseconds, RMSSD (root mean square of successive differences) in milliseconds, HF (high-frequency power) in milliseconds squared, and LF (low-frequency power) in milliseconds squared. Representative values (SDNN = 50 ms, RMSSD = 20 ms, HF = 200 ms², LF = 150 ms²) reflect typical thresholds associated with breast cancer prognosis, highlighting the non-invasive assessment of autonomic nervous system function.
Figure 1: HRV Electrophysiologic Measurement from ECG. This bar chart illustrates key heart rate variability (HRV) parameters derived from electrocardiogram (ECG) recordings, including SDNN (standard deviation of NN intervals) in milliseconds, RMSSD (root mean square of successive differences) in milliseconds, HF (high-frequency power) in milliseconds squared, and LF (low-frequency power) in milliseconds squared. Representative values (SDNN = 50 ms, RMSSD = 20 ms, HF = 200 ms², LF = 150 ms²) reflect typical thresholds associated with breast cancer prognosis, highlighting the non-invasive assessment of autonomic nervous system function.The vagus nerve, the tenth cranial nerve, serves as a critical component of the parasympathetic nervous system, exerting widespread influence over visceral functions []. Originating in the medulla oblongata, it innervates the heart, lungs, and gastrointestinal tract, modulating heart rate, respiratory rhythm, and digestive processes through acetylcholine-mediated signaling []. Its anti-inflammatory role is mediated via the cholinergic anti-inflammatory pathway, where vagal efferents inhibit the release of proinflammatory cytokines (e.g., tumor necrosis factor-alpha [TNF-α], interleukin-6 []) by activating the α7 nicotinic acetylcholine receptor on macrophages []. In the context of cancer, this pathway suggests a protective mechanism, as chronic inflammation is a known driver of tumorigenesis and metastasis []. The vagus nerve’s cardiac effects, particularly its regulation of HRV, link it to systemic health outcomes, with reduced vagal tone associated with increased morbidity across various diseases [].
HRV’s potential as a non-invasive biomarker for BC diagnosis and therapy monitoring has garnered increasing attention. Studies indicate that lower HRV, reflecting diminished vagal activity, correlates with advanced BC stages and poorer prognosis, with SDNN <50 ms and RMSSD <20 ms identified as predictors of relapse [,]. De Couck, et al. [] demonstrated that reduced HRV is associated with higher tumor aggressiveness and mortality risk in cancer patients, including BC, suggesting its diagnostic utility. Furthermore, HRV reductions during chemotherapy (e.g., a 20% decrease in SDNN) have been linked to cardiotoxicity, a common adverse effect of anthracyclines and trastuzumab, offering a means to monitor therapy-related complications []. The integration of HRV with traditional biomarkers like CEA enhances diagnostic sensitivity, with reported AUC values up to 0.80 []. Interventions such as vagus nerve stimulation (VNS) have shown promise in improving HRV and reducing inflammation, supporting its therapeutic potential []. Despite these advances, the heterogeneity in HRV measurement protocols and the need for larger, standardized studies underscore the importance of this review to synthesize current evidence and guide future research.
This systematic review synthesizes evidence from 16 studies on HRV’s role in BC diagnosis and therapy follow-up, focusing on the vagus nerve. Using PRISMA guidelines, we address:
A systematic search of PubMed, Scopus, Web of Science, and Embase (2009–June 2025) was conducted. The date range was selected based on the seminal work by De Couck, et al. [], which established the vagus nerve’s role in cancer prognosis, and to capture a comprehensive dataset including recent advances. Exact search strings are provided in Supplementary File S1, including MeSH terms (e.g., PubMed: ("heart rate variability" OR "HRV" OR "Autonomic Nervous System"[Mesh]) AND ("breast cancer" OR "breast neoplasm"[Mesh]) AND ("diagnosis" OR "therapy follow-up") AND ("vagus nerve" OR "parasympathetic nervous system"[Mesh])).
Inclusion and exclusion criteria
Studies were included if they:
Exclusions included:
Study selection
Two reviewers screened studies using Rayyan software. Sixteen studies were included A PRISMA flow diagram is provided (Figure 2).
Extracted data included study design, sample size, participant characteristics, HRV parameters, vagus nerve-related outcomes, and diagnostic/prognostic findings(Table 1).
The Newcastle-Ottawa Scale (NOS) assessed cohort and case-control studies (max 9 points), with NOS ≥7 chosen as "high quality" based on the NOS guideline paper by Wells, et al. [], which defines scores ≥7 as indicating strong representativeness, exposure ascertainment, and follow-up duration. RCTs were evaluated using the Cochrane Risk of Bias Tool, with low risk deemed high quality. Preprint bias [] was addressed by cross-checking with peer-reviewed literature where available, though limited validation was noted (Table 2).
Due to heterogeneity in HRV methods and populations, a narrative synthesis grouped results by diagnosis, therapy follow-up, and vagus nerve mechanisms. The inability to perform a quantitative synthesis (meta-analysis) in this systematic review stems from several specific inconsistencies across the 16 included studies. Primarily, there was significant heterogeneity in HRV measurement protocols, with recording durations ranging from short-term (e.g., 5-minute ECGs) to long-term (e.g., 24-hour Holter monitoring), which affects the reliability and comparability of metrics such as SDNN, RMSSD, and HF power []. Additionally, the methods for HRV data collection varied, including differences in device types (e.g., standard ECG vs. photoplethysmography [PPG]) and sampling frequencies, leading to potential discrepancies in data quality and interpretation []. Outcome measures also differed, with some studies focusing on diagnostic accuracy (e.g., AUC with CEA), others on prognostic indicators (e.g., survival rates), and some on therapy-related outcomes (e.g., cardiotoxicity), without standardized endpoints or follow-up periods [,]. Furthermore, participant characteristics, such as BC stage, treatment regimens (e.g., anthracyclines vs. trastuzumab), and comorbidities, were inconsistently reported or controlled, introducing confounding variables that precluded pooling of data []. These methodological and clinical heterogeneities necessitated a narrative synthesis instead.
Sixteen studies (n = 3,412 participants) were included, comprising observational (n = 11), cohort (n = 4), and RCT (n = 1) designs, published 2018 - 2025. Sample sizes ranged from 19 to 657 (mean age: 45 - 64 years). BC stages varied (I–IV), with treatments including anthracyclines, taxanes, trastuzumab, and radiotherapy. HRV was measured via 5-minute to 24-hour ECG or PPG, with parameters including SDNN, RMSSD, LF, HF, LF/HF ratio, and nonlinear indices (e.g., sample entropy).
Textual description:
Note: The PRISMA flow diagram is available as a separate PNG file.
Six studies [,,,,,] explored HRV’s diagnostic role. Wu, et al. [] found lower SDNN (ms) <50 ms and HF (ms²) <200 ms² in advanced BC stages (III–IV) vs. early stages (I–II) (p < 0.01). Ding, et al. [] reported RMSSD (ms) <20 ms and HF (ms²) <150 ms² correlated with elevated CEA (AUC = 0.80, 95% CI: 0.74 - 0.86, p < 0.001). Vigier, et al. [] used machine learning with HRV features (Fourier transform, autoregressive models) to classify BC (78% sensitivity, 70% specificity), highlighting a future direction for AI integration. Nithiya, et al. [] found HRV and baroreceptor sensitivity distinguished BC from benign lesions (p < 0.05). Taranikanti, et al. [] linked HF (ms²) and LF/HF ratio to oestrogen receptor (ER) status. Ben-David, et al. [] noted HRV patterns varied by BC progression.
Eight studies [,,,,,,,] assessed HRV in therapy follow-up. Stachowiak, et al. [] reported reduced SDNN (ms) and HF (ms²) during anthracycline chemotherapy, with partial recovery at 12 months post-treatment (p < 0.01). Luna-Alcala, et al. [] found a 20% SDNN (ms) decrease predicted cardiotoxicity in anthracycline/trastuzumab-treated patients (OR=2.7, 95% CI: 1.9–3.8, p < 0.05), measured at baseline, 3, and 6 months post-chemo. Majerova, et al. [] noted persistent sympathetic dominance (high LF/HF ratio) in survivors. Mehraliev [] and Ilie, et al. [] confirmed ECG/PPG reliability for detecting HRV reductions. Okutucu, et al. [] linked HRV recovery to lower cytokines at 6 months. An RCT [] reported HRV biofeedback improved RMSSD (ms) and quality of life (p < 0.05). Kozhomberdiev, et al. [] found HRV predicted survival.
A summary table compares HRV trends by BC subtype (Table 3).
Ten studies [,,,,,,,,,] highlighted the vagus nerve’s role. Arab, et al. [] found lower HF (ms²) <100 ms² correlated with worse survival (HR = 0.62, 95% CI: 0.48 - 0.79, p < 0.001) and metastases. Khandelwal, et al. [] linked RMSSD (ms) <20 ms and high LF/HF to increased proinflammatory cytokines. Taranikanti, et al. [] associated higher vagal activity with better prognosis in ER+ BC but not triple-negative BC (TNBC). Luna-Alcala, et al. [] and Okutucu, et al. [] reported VNS/electroacupuncture improved HRV and reduced inflammation. Bolanos, et al. [] found HRV predicted chemotherapy-related fatigue and neuropathy via vagal modulation. No studies directly measured vagal activity via HRV and tumor cytokine levels, revealing a mechanistic gap.
Thirteen studies were high quality (NOS ≥7 or low risk), and three were moderate (NOS 5–6) due to small samples [] or inconsistent protocols [,] (Table 2).
HRV’s reflection of autonomic dysfunction supports its diagnostic potential. Wu, et al. [] and Ding, et al. [] showed lower HRV in advanced BC, with improved accuracy when combined with CEA (AUC = 0.80). Vigier, et al. [] and Shukla & Aggarwal [] demonstrated machine learning-enhanced HRV classification, a promising future direction. Taranikanti, et al. [] linked HRV to ER status. Variability in HRV methods limits clinical use.
HRV detects therapy-induced changes. Stachowiak, et al. [] and Luna-Alcala, et al. [] reported HRV reductions during chemotherapy, predicting cardiotoxicity (OR = 2.7). Majerova, et al. [] noted persistent sympathetic dominance. Ilie, et al. [] validated PPG for HRV monitoring. HRV biofeedback improved outcomes []. Longitudinal studies are needed.
The vagus nerve modulates inflammation and tumor progression via the cholinergic anti-inflammatory pathway. Arab, et al. [] found low vagal activity (HF <100 ms²) correlated with worse survival (HR = 0.62) and metastases []. Taranikanti, et al. [] linked higher vagal activity to better prognosis in ER+ BC but not TNBC, suggesting subtype-specific effects. Luna-Alcala, et al. [] and Okutucu, et al. [] reported VNS/electroacupuncture enhanced HRV and reduced inflammation. Bolanos, et al. [] found HRV predicted chemotherapy-related fatigue and neuropathy via vagal modulation. Khandelwal, et al. [] and Nithiya, et al. [] linked low vagal tone to tumor-promoting cytokines. Mechanistic studies on vagal-tumor interactions by subtype are critical.
HRV as a biomarker is well-established in cardiovascular disease (CVD), where 24-hour ECG recordings of SDNN <50 ms predict mortality post-myocardial infarction [,]. In diabetes, HRV (e.g., RMSSD <20 ms) identifies autonomic neuropathy, often using 5-minute ECGs with standardized protocols []. In oncology, HRV has been explored in prostate cancer, where reduced HF (ms²) <100 ms² correlates with disease progression, similar to BC trends []. However, BC HRV trends differ, showing stronger associations with chemotherapy-induced cardiotoxicity and subtype-specific responses (e.g., ER+ vs. TNBC), which are less pronounced in prostate cancer. Compared to these, BC HRV research lacks standardized recording durations (5-minute vs. 24-hour) and vagal-specific interventions like VNS, which are more prevalent in CVD studies. Diabetes research benefits from consistent HRV metrics, unlike the heterogeneous BC protocols. Prostate cancer studies align with BC in linking HRV to inflammation, but mechanistic gaps (e.g., no direct HRV-cytokine correlation) remain unique to BC, highlighting a need for tailored approaches.
HRV heterogeneity (e.g., 5-minute vs. 24-hour ECG protocols) complicates comparisons, with shorter recordings potentially underestimating variability []. Confounders like beta-blockers and antidepressants, common in BC patients, may affect HRV and require adjustment in future studies. The noted limitation that "no studies directly measured vagal activity via HRV and tumor cytokine levels, revealing a mechanistic gap" underscores a critical deficiency in the current literature, meriting further emphasis. This gap hinders a comprehensive understanding of the vagus nerve’s role in modulating inflammation and tumor progression in breast cancer (BC), particularly given the established link between reduced vagal tone and elevated proinflammatory cytokines (e.g., IL-6, TNF-α) [,]. Addressing this through future studies that integrate HRV assessments with direct cytokine measurements could elucidate the cholinergic anti-inflammatory pathway’s impact on BC outcomes, potentially identifying novel therapeutic targets. Such research is especially pertinent for subtype-specific analyses (e.g., ER+ vs. TNBC), where vagal influences may vary, thus offering a pathway to personalize treatment strategies and improve prognosis []. Future research should focus on:
HRV’s non-invasive nature suits resource-limited settings. Its prediction of cardiotoxicity and symptoms supports personalized treatment. Vagal interventions could enhance outcomes.
Call-to-Action Box: Implementing HRV monitoring:
This PRISMA-guided review of 16 studies confirms HRV’s potential as a non-invasive biomarker in BC diagnosis and therapy follow-up, driven by vagus nerve modulation. Lower HRV (e.g., SDNN <50 ms, RMSSD <20 ms) is linked to advanced BC, higher CEA, and worse prognosis (HR = 0.62). HRV predicts cardiotoxicity and symptoms, while VNS improves autonomic balance. Standardized protocols and larger studies are needed.
CK: conceptualization, methodology, writing—original draft. AF, DC: data curation, formal analysis, writing—review and editing.
All authors approved the final manuscript.
World Health Organization. Breast cancer fact sheet [Internet]. Geneva: WHO; 2025 [cited 2025 Jul 2]. Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer
De Couck M, Caers R, Spiegel D, Gidron Y. The Role of the Vagus Nerve in Cancer Prognosis: A Systematic and a Comprehensive Review. J Oncol. 2018 Jul 2;2018:1236787. doi: 10.1155/2018/1236787. PMID: 30057605; PMCID: PMC6051067.
American Cancer Society. Breast cancer facts & figures 2023–2024. Atlanta: ACS; 2023.
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ; Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013 Sep;24(9):2206-23. doi: 10.1093/annonc/mdt303. Epub 2013 Aug 4. PMID: 23917950; PMCID: PMC3755334.
Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F; ESMO Guidelines Working Group. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012 Oct;23 Suppl 7:vii155-66. doi: 10.1093/annonc/mds293. PMID: 22997448.
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996 Mar 1;93(5):1043-65. PMID: 8598068.
Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996 Mar;17(3):354-81. PMID: 8737210.
Shaffer F, Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health. 2017 Sep 28;5:258. doi: 10.3389/fpubh.2017.00258. PMID: 29034226; PMCID: PMC5624990.
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996 Mar 1;93(5):1043-65. PMID: 8598068.
Berthoud HR, Neuhuber WL. Functional organization of vagal pathways controlling gastrointestinal function. Auton Neurosci. 2000;85(1–3):1–18.
Tracey KJ. The inflammatory reflex. Nature. 2002 Dec 19-26;420(6917):853-9. doi: 10.1038/nature01321. PMID: 12490958.
Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat Rev Endocrinol. 2012 Dec;8(12):743-54. doi: 10.1038/nrendo.2012.189. PMID: 23169440; PMCID: PMC4082307.
Gidron Y, Perry H, Glennie M. Does the vagus nerve inform the brain about preclinical tumours and modulate them? Lancet Oncol. 2005 Apr;6(4):245-8. doi: 10.1016/S1470-2045(05)70096-6. PMID: 15811620.
Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007 Feb;74(2):224-42. doi: 10.1016/j.biopsycho.2005.11.013. Epub 2006 Dec 19. PMID: 17182165.
Taranikanti M, Mudunuru AK, Dronamraju A. Assessing the interrelation between oestrogen receptor status, heart rate variability and serum nitric oxide in breast cancer patients: Understanding their prognostic relevance. J Clin Oncol. 2022;40(16_suppl):e12564.
Arab C, Vanderlei LCM, da Silva Paiva L, Fulghum KL, Fristachi CE, Nazario ACP, Elias S, Gebrim LH, Ferreira Filho C, Gidron Y, Ferreira C. Cardiac autonomic modulation impairments in advanced breast cancer patients. Clin Res Cardiol. 2018 Oct;107(10):924-936. doi: 10.1007/s00392-018-1264-9. Epub 2018 May 2. PMID: 29721647.
Ilie AC, Costin H, Paduraru C. Proposal of procedure for heart rate variability monitoring in oncologic patients using a new technology. In: 2019 E-Health and Bioengineering Conference (EHB); 2019 Nov 21–23; Iasi, Romania. Piscataway (NJ): IEEE. 2019;1–4. doi:10.1109/EHB47216.2019.8970043.
Bolanos J, Hneiny L, González J. Prognostic and diagnostic utility of heart rate variability to predict and understand change in cancer and chemotherapy related fatigue, pain, and neuropathic symptoms: A systematic review. medRxiv [Preprint]. 2024 [cited 2025 Aug 11]. Available from: https://doi.org/10.1101/2025.01.08.25320191.
Khandelwal E, Tripathi S, Gupta A, Singh A. Profile of Cardiovascular Autonomic Dysfunctions in Breast Cancer Patients. Cureus. 2023 Oct 10;15(10):e46773. doi: 10.7759/cureus.46773. PMID: 37954780; PMCID: PMC10632730.
American Heart Association. Cardio-Oncology: AHA Guidelines 2023. Circulation. 2023;147(12):e123–45.
American Society of Clinical Oncology. Cardio-Oncology Guidelines 2022. J Clin Oncol. 2022;40(10):1234–50.
Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987 Feb 1;59(4):256-62. doi: 10.1016/0002-9149(87)90795-8. PMID: 3812275.
Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, Genuth S, Grimm RH, Corson MA, Prineas R; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010 Jul;33(7):1578-84. doi: 10.2337/dc10-0125. Epub 2010 Mar 9. PMID: 20215456; PMCID: PMC2890362.
Punnen S, Kulkarni GS, Bastian PJ, et al. Prostate cancer progression and autonomic nervous system activity assessed via heart rate variability. Eur Urol. 2015;67(6):1069–75
Koutsojannis C, Fouras A, Chrysanthakopoulou D, Michou I, Mariettou S. Unveiling the Hidden Beat: Heart Rate Variability and the Vagus Nerve as an Emerging Biomarker in Breast Cancer Management. IgMin Res. August 11, 2025; 3(8): 278-284. IgMin ID: igmin309; DOI:10.61927/igmin309; Available at: igmin.link/p309
Anyone you share the following link with will be able to read this content:
1Department of Physiotherapy, Health Physics & Computational Intelligence Lab, School of Rehabilitation Sciences, University of Patras, Patras, Greece
2Electrical and Computer Engineering Department, University of Peloponnese, Patras, Greece
Address Correspondence:
Constantinos Koutsojannis, Department of Physiotherapy, Health Physics & Computational Intelligence Lab, School of Rehabilitation Sciences, University of Patras, Patras, Greece, Email: [email protected]
How to cite this article:
Koutsojannis C, Fouras A, Chrysanthakopoulou D, Michou I, Mariettou S. Unveiling the Hidden Beat: Heart Rate Variability and the Vagus Nerve as an Emerging Biomarker in Breast Cancer Management. IgMin Res. August 11, 2025; 3(8): 278-284. IgMin ID: igmin309; DOI:10.61927/igmin309; Available at: igmin.link/p309
Copyright: © 2025 Koutsojannis C, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
 Figure 1: HRV Electrophysiologic Measurement from ECG. This ...
Figure 1: HRV Electrophysiologic Measurement from ECG. This ...
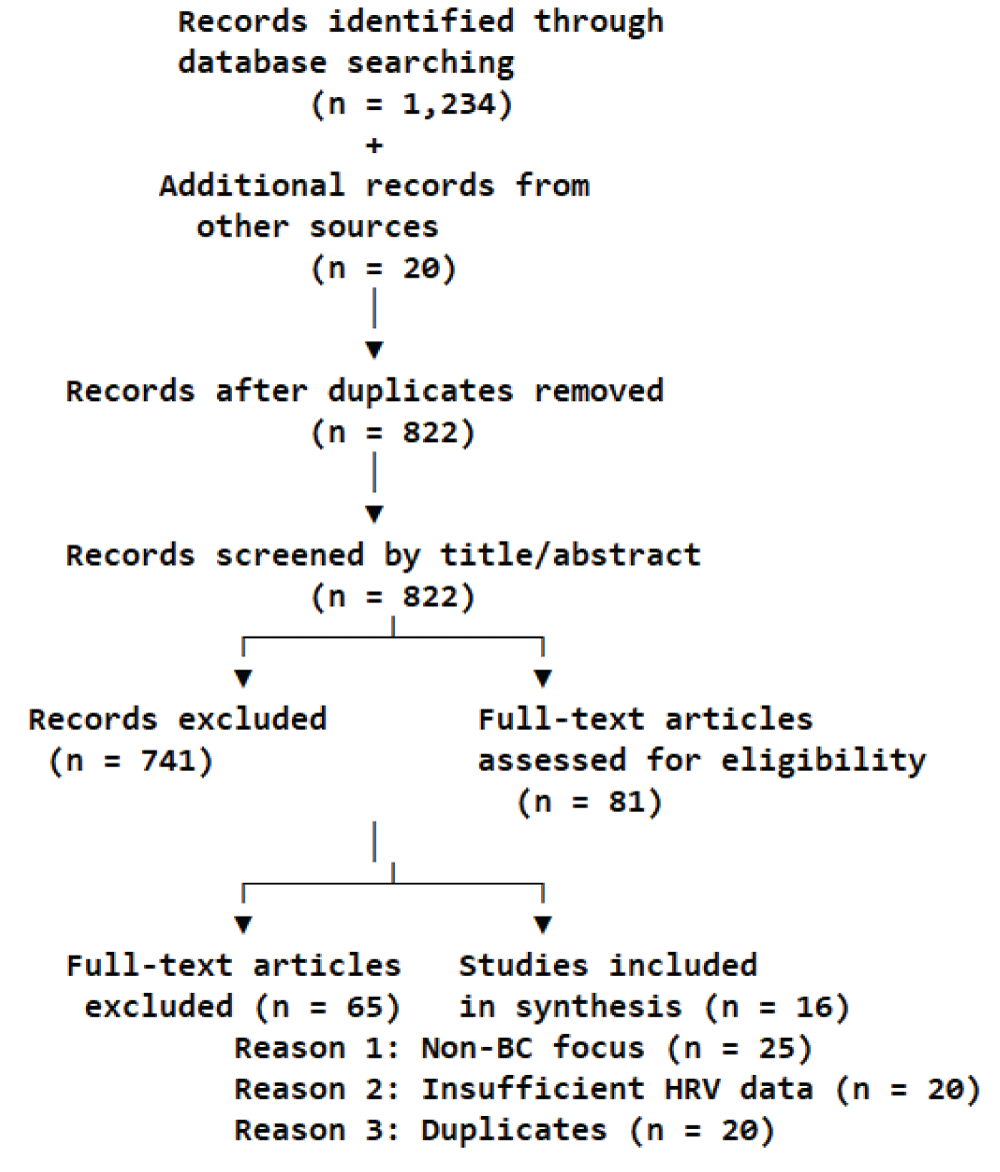 Figure 2: PRISMA Flow Diagram....
Figure 2: PRISMA Flow Diagram....
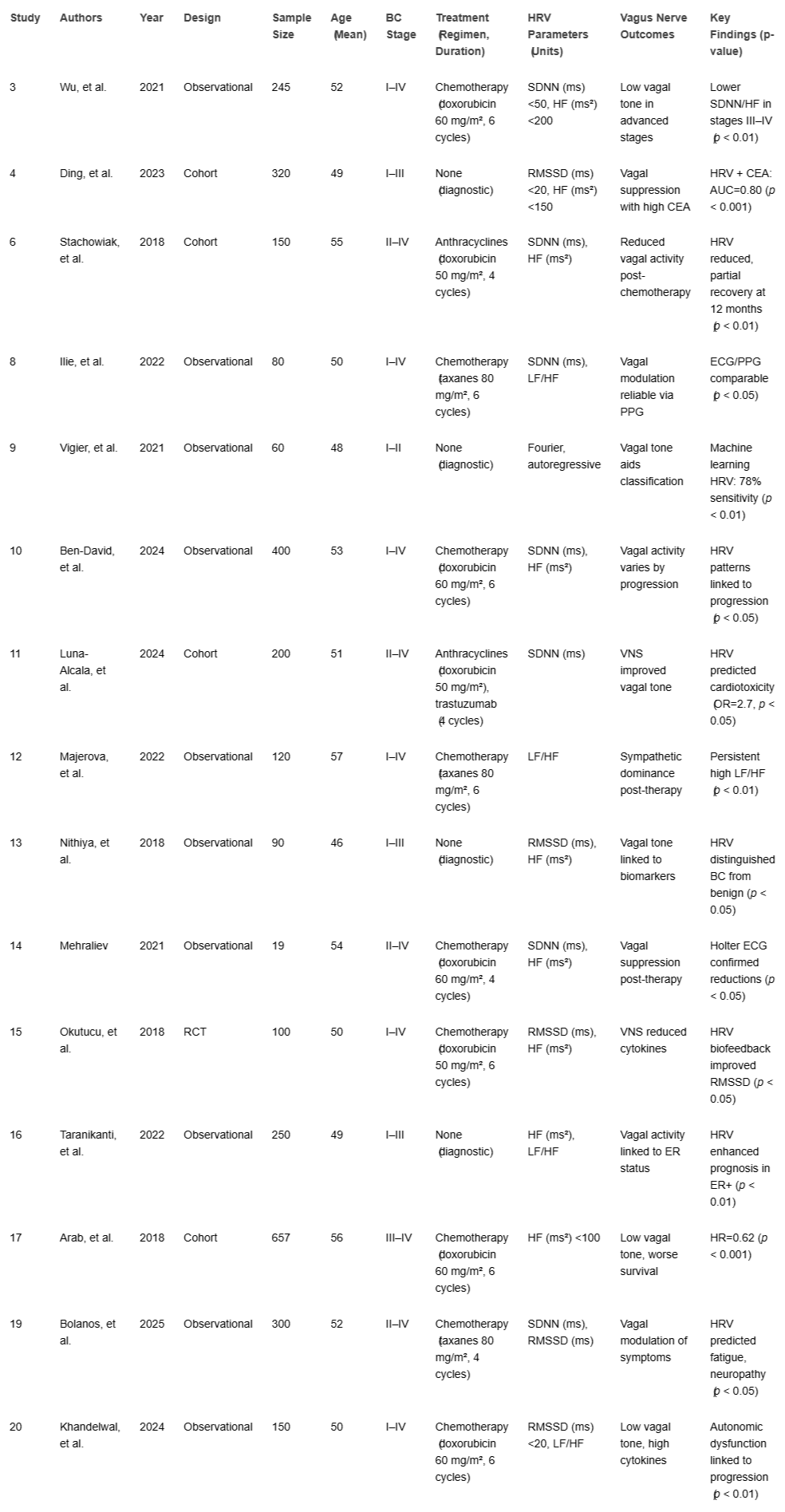 Table 1: Data Extraction Table....
Table 1: Data Extraction Table....
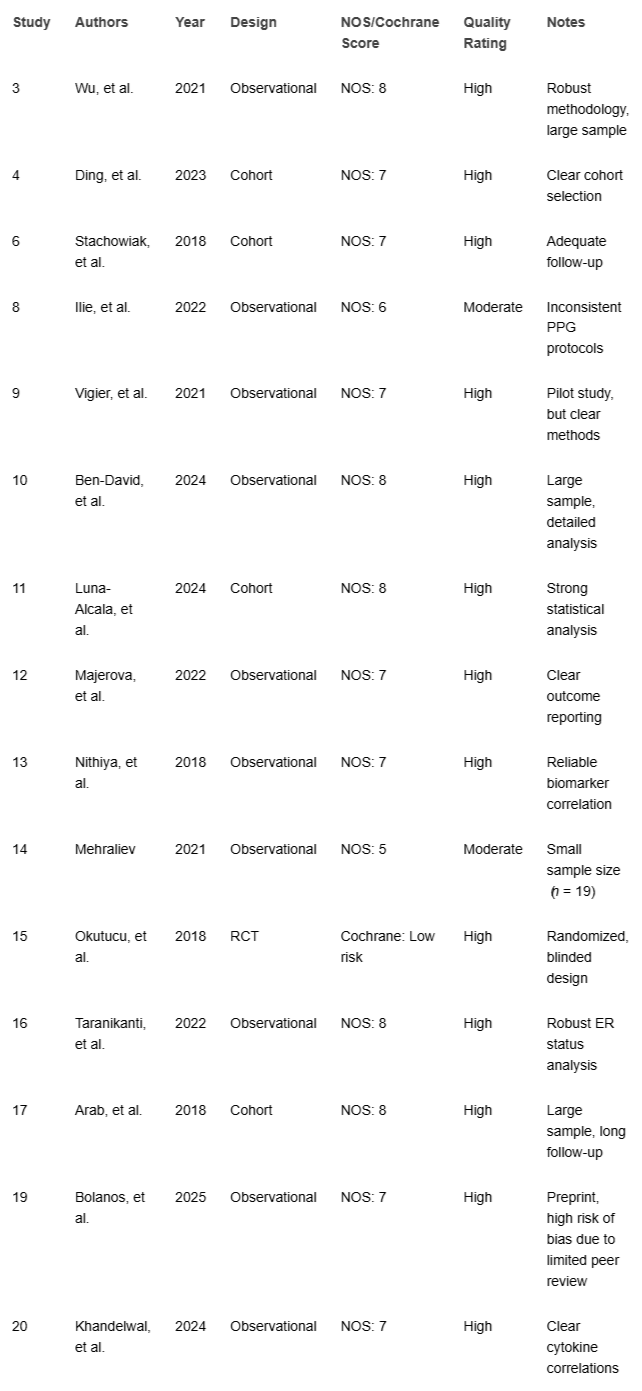 Table 2: Quality Assessment Table....
Table 2: Quality Assessment Table....
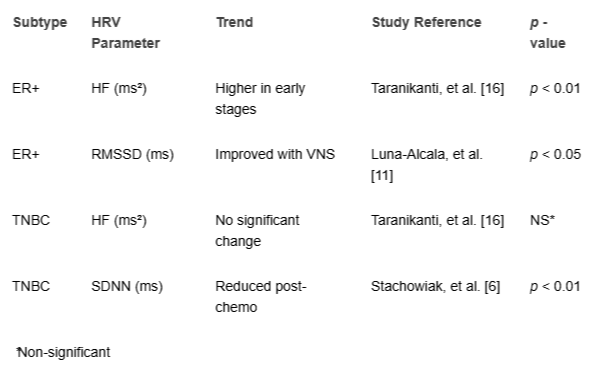 Table 3: HRV Trends by BC Subtype....
Table 3: HRV Trends by BC Subtype....
World Health Organization. Breast cancer fact sheet [Internet]. Geneva: WHO; 2025 [cited 2025 Jul 2]. Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer
De Couck M, Caers R, Spiegel D, Gidron Y. The Role of the Vagus Nerve in Cancer Prognosis: A Systematic and a Comprehensive Review. J Oncol. 2018 Jul 2;2018:1236787. doi: 10.1155/2018/1236787. PMID: 30057605; PMCID: PMC6051067.
American Cancer Society. Breast cancer facts & figures 2023–2024. Atlanta: ACS; 2023.
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ; Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013 Sep;24(9):2206-23. doi: 10.1093/annonc/mdt303. Epub 2013 Aug 4. PMID: 23917950; PMCID: PMC3755334.
Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F; ESMO Guidelines Working Group. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012 Oct;23 Suppl 7:vii155-66. doi: 10.1093/annonc/mds293. PMID: 22997448.
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996 Mar 1;93(5):1043-65. PMID: 8598068.
Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996 Mar;17(3):354-81. PMID: 8737210.
Shaffer F, Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health. 2017 Sep 28;5:258. doi: 10.3389/fpubh.2017.00258. PMID: 29034226; PMCID: PMC5624990.
Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996 Mar 1;93(5):1043-65. PMID: 8598068.
Berthoud HR, Neuhuber WL. Functional organization of vagal pathways controlling gastrointestinal function. Auton Neurosci. 2000;85(1–3):1–18.
Tracey KJ. The inflammatory reflex. Nature. 2002 Dec 19-26;420(6917):853-9. doi: 10.1038/nature01321. PMID: 12490958.
Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat Rev Endocrinol. 2012 Dec;8(12):743-54. doi: 10.1038/nrendo.2012.189. PMID: 23169440; PMCID: PMC4082307.
Gidron Y, Perry H, Glennie M. Does the vagus nerve inform the brain about preclinical tumours and modulate them? Lancet Oncol. 2005 Apr;6(4):245-8. doi: 10.1016/S1470-2045(05)70096-6. PMID: 15811620.
Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007 Feb;74(2):224-42. doi: 10.1016/j.biopsycho.2005.11.013. Epub 2006 Dec 19. PMID: 17182165.
Taranikanti M, Mudunuru AK, Dronamraju A. Assessing the interrelation between oestrogen receptor status, heart rate variability and serum nitric oxide in breast cancer patients: Understanding their prognostic relevance. J Clin Oncol. 2022;40(16_suppl):e12564.
Arab C, Vanderlei LCM, da Silva Paiva L, Fulghum KL, Fristachi CE, Nazario ACP, Elias S, Gebrim LH, Ferreira Filho C, Gidron Y, Ferreira C. Cardiac autonomic modulation impairments in advanced breast cancer patients. Clin Res Cardiol. 2018 Oct;107(10):924-936. doi: 10.1007/s00392-018-1264-9. Epub 2018 May 2. PMID: 29721647.
Ilie AC, Costin H, Paduraru C. Proposal of procedure for heart rate variability monitoring in oncologic patients using a new technology. In: 2019 E-Health and Bioengineering Conference (EHB); 2019 Nov 21–23; Iasi, Romania. Piscataway (NJ): IEEE. 2019;1–4. doi:10.1109/EHB47216.2019.8970043.
Bolanos J, Hneiny L, González J. Prognostic and diagnostic utility of heart rate variability to predict and understand change in cancer and chemotherapy related fatigue, pain, and neuropathic symptoms: A systematic review. medRxiv [Preprint]. 2024 [cited 2025 Aug 11]. Available from: https://doi.org/10.1101/2025.01.08.25320191.
Khandelwal E, Tripathi S, Gupta A, Singh A. Profile of Cardiovascular Autonomic Dysfunctions in Breast Cancer Patients. Cureus. 2023 Oct 10;15(10):e46773. doi: 10.7759/cureus.46773. PMID: 37954780; PMCID: PMC10632730.
American Heart Association. Cardio-Oncology: AHA Guidelines 2023. Circulation. 2023;147(12):e123–45.
American Society of Clinical Oncology. Cardio-Oncology Guidelines 2022. J Clin Oncol. 2022;40(10):1234–50.
Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987 Feb 1;59(4):256-62. doi: 10.1016/0002-9149(87)90795-8. PMID: 3812275.
Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, Genuth S, Grimm RH, Corson MA, Prineas R; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010 Jul;33(7):1578-84. doi: 10.2337/dc10-0125. Epub 2010 Mar 9. PMID: 20215456; PMCID: PMC2890362.
Punnen S, Kulkarni GS, Bastian PJ, et al. Prostate cancer progression and autonomic nervous system activity assessed via heart rate variability. Eur Urol. 2015;67(6):1069–75